Hot and Stinky: the Oceans without Oxygen
Contents:
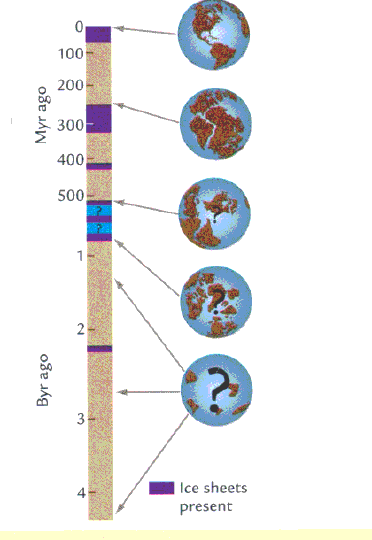
We live in an unusual time of Earth history, because there are large ice sheets in the northern and southern polar areas. Considerable evidence from sedimentary rocks and from fossils demonstrates that the Earth usually lacked large ice sheets, even in the polar regions. The Earth's climate has thus alternated over time between times during which ice sheets existed at high latitudes (Icehouse Earth), and periods where ice sheets were essentially absent (Greenhouse Earth).
Figure from W. F. Ruddiman, 2001, Earth's Climate: Past and Future. (W. H. Freeman & Co), p. 84. 'During Earth's 4.55 billion years history, intervals when large continental ice sheets were present alternated with times when they were not (left). The earliest history of these changes is poorly defined because few ancient records are preserved. The movements of continents in relation to ocean basins are well known only for the last several hundred million years (right).'

We do not know what causes these swings in the Earth's climate. The position of the continents may play a role: polar ice sheets can form only when there are continents close to the poles. The position of the continents also limits the patterns of ocean surface currents, which can transport heat from low to high latitude. We think, however, that in many cases the amount of carbon dioxide in the atmosphere may have played an important role. During times when huge flood basalts formed, for instance, large amounts of carbon dioxide from such flows reached the atmosphere, and caused warming. During times when continents collided (such as India with the Asian mainland), large mountain ranges formed (such as the Himalayas). During the extensive weathering of the mountain ranges much carbon dioxide was taken out of the atmosphere for the weathering reaction, and atmospheric levels of carbon dioxide dropped, leading to global cooling.
2. The Biosphere and Global Climate Changes
Biospheric processes were of major importance for the carbon cycle. During times when large deposits of coal or oil were formed, the carbon dioxide which was used to make the organic matter in photosynthesis was taken out of the atmospheric and buried in the rocks (lithosphere), causing global cooling. Major events in the history of life thus may have had a large effect on global climate.
The evolution of burrowing animals at the beginning of the Cambrian period (550 million years ago) may have influenced the recycling of carbon from the ocean floor into the ocean waters, as discussed under 'Snowball Earth'.
Evolution of land plants, followed by the establishment of the first forests in the late Devonian (about 415 million years ago) probably had a large effect. When these forests evolved, many logs were buried in the sediments, forming large coal deposits. We think that many of these logs were buried because insects that can digest wood using bacteria in their guts had not yet evolved, so that wood did not rot easily. Much CO2 was taken out of the atmosphere (present in the organic matter of the coal), and the amount of oxygen gas in the atmosphere increased. Giant insects then evolved; insects have no lungs, breathe through little tubes in their body, and are limited in size because oxygen moves slowly inside these tubes. Large insects can not survive and suffocate because of a lack of oxygen. But under high-oxygen conditions in the atmosphere giant insects could evolve, who became extinct when oxygen levels dropped again. The world underwent a severe cooling at the end of the Carboniferous (coal-forming) period.
3. The Rocks as Recorders of Global Climate
How can we judge whether ice sheets occurred on Earth? We can recognize rocks which indicate that glaciers are close. In the oceans, we use the occurrence of ice-rafted material, including drop stones. On land, we use various types of rocks, including rocks that were scratched by glaciers, and mixture of coarse boulders, sand and clay that were dropped at the end of glaciers by the melting ice. We also can recognize sediments deposited in lakes close to glaciers.
We need information on the age of rocks showing evidence for the existence of ice sheets, as well as for their latitude and altitude (distance from sea level; after all, high up in the mountains we find glaciers even in the tropics). If we find evidence for the existence of ice sheets close to sea level at high latitudes, we know that polar ice caps existed. If we find evidence for the existence of ice sheets at sea level even at low latitudes, we know that we have a very unusual situation: Snowball Earth.
The oldest period of Earth history for which we find evidence for the existence of ice sheets occurred about 2914-2990 million years ago, in the period called Middle Archean. We have a very limited rock record from rocks that are so old, and do not know how extensive glaciation was at the time. A second period of glaciation occurred in the period called Proterozoic, between 2400 to 2200 million years ago. This glaciation was recognized on several continents. We have no records at all of the occurrence of ice sheets between 2200 and 950 million years ago.
Between 520 and 950 million years ago there were several ice ages (probably 2 to 3), some of which were extremely severe (Snowball Earth), and may have influenced the evolution of animals on Earth. There was a fairly short and severe ice age in the Ordovician-Silurian period (445 to 429 million years ago), and another one in the late Devonian through early Carboniferous (363 to 353 Ma). During the later part of the Paleozoic (338 to 256 Ma) ice sheets waxed and waned, and climate showed strong fluctuations. Note that ice sheets had disappeared by the end of the Pemrian at 250 Million years. But during the whole of the Mesozoic (the time of the dinosaurs) climate was dominantly warm, with possibly a few short periods during at least small ice caps may have existed, but no major ice sheets.
The world started to cool once again in the Cenozoic, towards the end of the Eocene time period at about 40 to 45 million years ago, which will be the topic of next week's discussions. Today's discussion concerns the long period of warmth of the Mesozoic, during which period all our most extensive oil reserves formed.
4. The Oceans in Warm and Cold Climates
In the present day oceans, the waters at the bottom are very cold (close to freezing). This is valid for waters all over the Earth: deep waters are cold all over the world, although the surface temperature of the oceans varies from close to freezing at the poles to about 30oC at the equator. This temperature pattern is caused by the fact that the deep waters fill the oceans at the poles: at these high latitudes the waters are very cold. If sea-ice forms (which is not salty because the salt remains behind when sea water freezes), the remaining water is very cold as well as very salty. Cold and salty water has a high density, and sinks to the ocean floors. Deep waters stream from the North Atlantic (close to Greenland) to the South Atlantic, where they mix with waters streaming out of the Weddell Sea (Antarctica). This cold mix streams around the Antarctic continent, with currents flowing over the bottom into the Indian and Pacific Oceans. It takes water that sinks in the northern Atlantic about 1000 years to reach the northern Pacific.
Upwelling of the deep waters occurs much more diffuse than sinking. The deep cold waters well up to the surface in several areas of upwelling, for instance in the eastern equatorial Pacific, along the coast of Peru, of Namibia, and off Oman. The deep cold water have been enriched in rotting organic matter (which falls to the bottom of the ocean), and they are thus enriched in nutrients (fertilizer). Where these waters well up, high primary productivity occurs in the surface waters. Such regions are the richest fisheries in the world.
In the present-day oceans, almost all deep waters are well oxygenated, with the exception of two types of environments: the Black Sea and the Oxygen Minimum Zones along the continents. We are looking at these environments when trying to understand sedimentation in the past. During warm periods of Earth History, however, we find indications of the widespread occurrence of anoxic conditions. Sediments deposited during such conditions are typically very dark brown to black, and show fine laminations (similar to the sediments deposited in lakes, seen on the field trip). The sediments have these laminations because no worms who dig through sediments and disturb lamination can live without oxygen.These fine-grained sediments are called black shales.
Such anoxic conditions in the oceans were particularly widespread during the Early Cretaceous, between about 85 and 125 million years ago. Periods of widespread occurrence of black shales are called Oceanic Anoxic Events (or OAEs). The best-documented OAEs occurred between 121 to119 Ma (OAE 1a in the early Aptian period), between 112 and109 Ma (OAE 1b in the early Albian period), between 102 and 98 Ma (OAEs 1c-1d in the late Albian period), and between 93 and 94 Ma (OAE 2 at the Cenomanian/Turonian boundary).
The two regions with low oxygen conditions in the oceans today are:
There has been and is a long-standing discussion on the origin of black shales, which have been studied extensively because of their economic value (oil). There is still no agreement, whether black shales reflect dominantly high productivity and are an ancient analog of oxygen minimum zones in the present oceans, or whether they reflect low productivity and restricted, sluggish circulation, and are an ancient analog of the Black Sea. Or maybe the ancient anoxic conditions have no counterpart in our present world, because the deep sea is now frigid. All ancient black shales conditions occurred during times that the oceans were very warm (as deduced from the oxygen isotopic composition of carbonates), up to 10 to 15oC warmer than today. This had many consequences.
Points 2 and 3 suggest that the overall productivity of the oceans was lower during warm periods. This point is still strongly debated: if oceanic productivity was so low, then how could all the organic matter that is preserved in oil be formed in the first place? There is thus no agreement on the question whether the ancient episodes of anoxia in the oceans reflect oceans resembling the Black Sea (very limited circulation) or resembling the present Oxygen Minimum Zones (good circulation but very high productivity).
5. How to start an Oceanic Anoxic Event
Oceanic Anoxic Events did not occur during human history, and not even during the whole of the Cenozoic (the last 65 million years). There is a possibility that at least one Cenozoic event represented an Oceanic Low-Oxygen Event (low oxygen instead of no oxygen). This event occurred in the Latest Paleocene, about 55.5 million years ago. This period was characterized by a very warm climate for the Cenozoic, and the absence of ice caps at the poles. Fossil crocodiles and palm trees have been found at very high northern latitudes (Spitsbergen Island, at >65oN). During this warm time period the mammals evolved rapidly, with groups such as the primates (including monkeys and us), the bats, the whales, and the hoofed mammals evolving.
In the deep sea, there was a sudden mass extinction of unicellular Eukaryotic organisms with a calcareous shell (foraminifera) living on the bottom of the oceans. The three figures below show Scanning Electron Micrographs of three species of foraminifera: Aragonia aragonensis, left, specimen length about 125 micrometer; Tappanina selmensis, middle, specimen length about 100 micrometer, and Gavelinella beccariiformis, right, specimen diameter about 350 micrometer. G. beccariiformis was a cosmopolitan, common species from Late Cretaceous through the Paleocene, survived the extinction of the dinosaurs and planktonic foraminifera at the end of the Cretaceous, but became extinct at the end of the Paleocene. It is typical for the 30-50% of species becoming extinct: large and heavily calcified. The other two species are opportunistic species which bloomed to great abundance after the extinction.
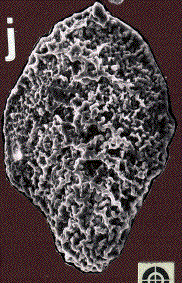
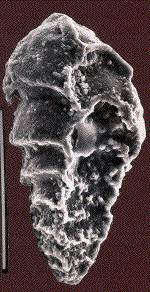
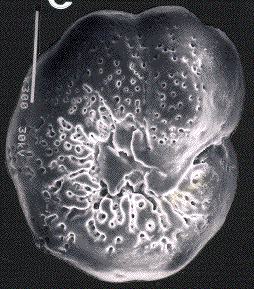
The extinction of the benthic foraminifera (including G. beccariiformis) at the end of the Paleocene occurred at the same time as a rapid warming of the deep-sea all over the world, by 4 to 6oC. A similar change was observed in the shells of planktonic foraminifera at middle to high latitudes, and surface waters at these latitudes thus also warmed, although temperatures did not change much in the tropics. This short period of extreme warming has been called the Paleocene-Eocene Thermal Maximum (abbreviated as LPTM).
During this warming (which can be seen clearly in the oxygen isotope records of benthic foraminifera) the carbon isotopes of these same benthic foraminifera show a large change to very low values (by more than 2.5 per mille). A similar change was seen in the records of planktonic foraminifera worldwide. In addition, a similar change was observed in land plant fossils, in teeth of herbivores, and in soil nodules formed on land. The abnormally light values of carbon isotopic composition thus were present throughout the ocean-atmosphere.
FIGURE (after Koch et al., 1992, Correlation between isotope records in marine and continental carbon reservoirs near the Palaeocene/Eocene boundary. Nature 358, p. 319-322). Transmission of changes in carbon isotopic composition throughout the ocean-atmosphere system: the various components of the system have different values, but the offsets between these values remain constant.
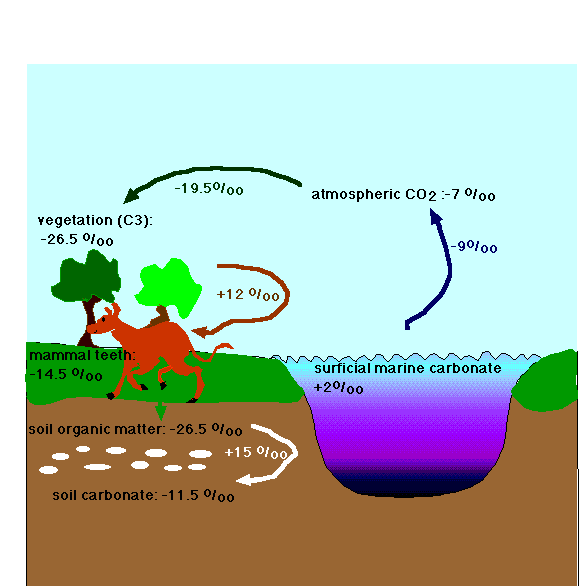
The change towards a much lower carbon isotopic composition of compounds formed on land (teeth, nodules) and in shells formed in the shallow as well as deep oceans worldwide must mean that a large amount of isotopically light carbon was let loose in the worlds' environments: a process similar in effect to the burning of large masses of fossil fuel. This addition of isotopically light carbon happened rapidly (in geological terms), i.e., over less than a few thousands of years.
The three 'usual suspects' for changes in the carbon isotopic composition of the atmosphere-ocean system thus were all out!
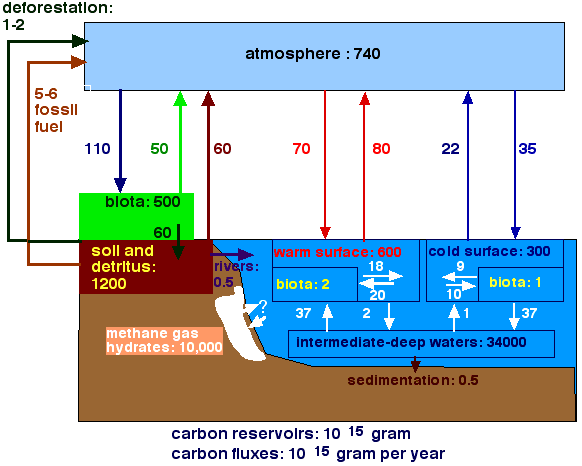
Therefore, there was a major problem in trying to explain this rapid change in the global carbon cycle, until the discovery of very large deposits of gas hydrates (also called clathrates). These gas hydrates are also called methane hydrates, because methane is the most common gas. The hydrates consist of ice, which is filled with methane gas. They occur in large quantities along the continental margins. They occur everywhere where the temperature is low enough, and/or pressure is high, and where methane and water are present. Under the sea, water is clearly present. The pressure comes from the overlying water masses. The methane is made by bacteria which use organic matter. Present estimates are that about 10,000 Gigaton of carbon is present in such gas hydrates. This is a huge amount (see figure below), about a third of all the carbon dissolved in the deep oceans, and much more than is present in the total biosphere, in soils, and in fossil fuel. We must update are ideas about the carbon cycle to include these large, until recently unsuspected carbon reservoir.
In the last few years, many governments (including those of the US, Canada, Russia, India and Japan) have become very interested in possibilities of methane hydrates as fossil fuels, but there are environmental concerns. The methane hydrates are stable under high pressure and low temperature. There is thus a possibility that they can dissociate, fall apart, and liberate their methane into the oceans, and from there into the atmosphere when pressure falls (sea level falls) or when temperature rises (and the temperature of the deep oceans is going up!). If global warming would result in warming of the oceans, for instance, methane hydrates might dissociate and get into the atmosphere. Methane is a potent greenhouse gas, and release of large amounts of methane into the atmosphere will result in more global warming. The methane will become oxidized to CO2 within about 10 years or so, but then the CO2 will contribute to global warming, which could lead to more dissociation of gas hydrates and more warming and so on: a typical example of destabilizing positive feedback. Presently, the potential of dissociation of gas hydrates as a result of future global warming is being investigated.
The carbon isotopic composition of methane in methane hydrates is very low (about minus 60 per mille on average). We can thus possibly recognize past blow-ups of large amounts of such gas hydrates in the rock record: they are represented by vary rapid and very large decreases in the carbon isotope record of foraminifera. We now have the following scenario for the events in the late Paleocene, about 55.5 million years ago:
We do not quite know why the warming stopped. Maybe the high latitudes became so warm that there were only very small temperature differences between the tropics and the high latitudes. That would have really slowed down winds and therefore also ocean currents, which would have slowed down heat transport to high latitudes. These high latitudes then cooled, and the cooler waters flowed to the bottom of the oceans, cooling them again. Maybe the oceanic organisms took up much carbon dioxide and oceanic productivity increased, thus counteracting the greenhouse effect. Maybe productivity on land increased, similarly taking CO2 out of the atmosphere.
Recently, detailed investigation of the carbon isotope records across interval of black shale deposition in the Cretaceous, long before these events in the late Paleocene, has shown a curious pattern in carbon isotopic records across the anoxic events; it has been well documented for OAE1b. The organic matter deposited in black shales has a light carbon isotopic signature, as has all organic matter (delta values around minus 23 per mille). During the deposition of such black shales we thus see an increase in the carbon isotopic signature of limestones (the lighter isotope is stored in the black shales, and the heavier one remains in the oceans, and is used during formation of carbonate). BUT just below the black shales and the interval with heavy carbon isotope values there was a very short, very large peak of very low carbon isotopic values.
It has now been suggested that this large, but short-lived change to very light carbon isotopic values represents release of gas hydrates in the environment. The release of gas hydrates then caused extreme warming (shown in the oxygen isotopic records), and the oxidation of the methane used up what little oxygen there was in the oceans. Then organic matter was stored in the oceanic sediments, because it could not rot in the absence of oxygen in the ocean waters. But this storage of organic matter also meant that carbon dioxide was once again taken out of the atmosphere, and the world cooled once again. And the cooling of the ocean waters brought oxygen back into the deep oceans.
Such a pattern has now been found in early Jurassic black shales (about 183 Ma) as well. Similarly, carbon isotopic excursions across the largest extinction of the last 540 years, the mass extinction at the end of the Permian, have been seen as indications that this extinction was triggered by methane release, which caused severe anoxia in the ocean. Note that during and just before the end-Permian extinction there were huge eruptions of flood basalts in Siberia, which could have led to the initial global warming by carbon dioxide emissions. Similarly, there were large flood basaltic eruptions in the Early Cretaceous just before and during the Oceanic Anoxic Events (e.g., the Ontong-Java Plateau in the Pacific Ocean), and in the early Jurassic (Karroo-Ferra basalts). The simple schedule outlined for the Late Paleocene event (above) thus may have validity to explain events of much more severe anoxia in the past.
In conclusion, the Earth's has alternated between warm periods ('greenhouse Earth') and cold periods ('ice house Earth'). We are now in the ice house; green house periods have been much more common than ice house periods over all of Earth History. During warm periods there were no polar ice caps, the continents were flooded and shallow seas (commonly with reefs) were extensive. The oceans contained little dissolved oxygen, and oceanic primary productivity may have been low, on average. The species diversity of oceanic organisms was high during these times of low productivity. Cold periods (such as we have now) characteristically have large ice caps, low sea level and relatively few shallow seas, high primary oceanic productivity but relatively low species diversity. During the warm periods, the dissociation of gas hydrates may have caused climatic instability, extreme warming, and anoxia.
7. Figure of the Carbon Cycle incorporating Gas Hydrates
This field of research develops very rapidly. Here are some recent references; you can also look in the book edited by M.-P. Aubry, S. Lucas, and W. A. Berggren, eds., Late Paleocene-early Eocene Biotic and Climatic Events in the Marine and Terrestrial Records, Columbia University Press, and you can look at the most recent volume of the quarterly journal of The Geological Society of Sweden (GFF vol. 122, part 1, pages 1-192), which shows extended abstracts of a meeting held in June 1999.
Bains, S., Corfield, R. M., and Norris, R. D., 1999. Mechanisms of Climate Warming at the End of the Paleocene. Science, 285, 724-727.
Bains, S., Norris, R. D., Corfield, R. M., and Faul, K., 2000. Termination of global warmth at the Palaeocene/Eocene boundary through productivity feedback. Nature, 407, 171-174.
Beerling, D., 2000. Increased terrestrial carbon storage across the Palaeocene Eocene boundary. Palaeogeography, Paleoclimatology, Palaeoecology, 161, 395-405.
Dickens, G. R., 1999. The blast in the past. Nature, 401, p. 752-853
Dickens, G. R., 2000. Methane oxidation during the Late Palaeocene Thermal Maximum. Bull. Soc. Geol. France, 171, 37-49.
Hesselbo, S. P., Groecke, D. R., Jenkyns, H. C., Bjerrum, C. J., Farrimond, P., Morgans Bell, H. S., and Green, O., 2000. Massive dissociation of gas hydrates during a Jurassic anoxic event. Nature, 406, 392-395.
Katz, M.E., Pak, D.K., Dickens, G.R., Miller, K.G., 1999. The source and fate of massive carbon input during the latest Paleocene thermal maximum. Science, 286, 5444, 1531-1533.
Koch, P. L., Zachos, J. C., and Gingerich, P. D., 1992, Coupled Isotopic Changes in Marine and Continental Carbon Reservoirs at the Paleocene-Eocene Boundary. Nature, 358, 319-322
Larson, R. L., and Erba, E., 1999. Onset of the mid-Cretaceous greenhouse in the Barremian-Aptian: igneous events and the biological, sedimentary, and gechemical consequences. Paleoceanography, 14, 663-678.
Norris R. D, and Röhl, U., 1999, Carbon cycling and chronology of climate warming during the Palaeocene/Eocene transition. Nature, 401, 775-778.
Röhl, U., Bralower, T. J., Norris, R. D., and Wefer, G., 2000. A new chronology for the late Paleocene thermal maximum and its environmental implications. Geology, 28: 927-930.
Thomas, E., 1998, The biogeography of the late Paleocene benthic foraminiferal extinction, In: M.-P. Aubry, S. Lucas, and W. A. Berggren, eds., Late Paleocene-early Eocene Biotic and Climatic Events in the Marine and Terrestrial Records, Columbia University Press, 214-243
Thomas, E., Zachos, J. C., and Bralower, T. J., 1999, Deep-Sea Environments on a Warm Earth: latest Paleocene - early Eocene. B. Huber, K. MacLeod, and S. Wing eds., Warm Climates in Earth History, Cambridge University Press, 132-160.
Zachos, J.C., Lohmann, K.C., Walker, J.C.G., and Wise, S.W., 1993. Abrupt climate change and transient climates during the Paleogene: A marine perspective. Journal of Geology, 101, 191-213