Basic data on carbon isotope analysis and the basic concepts are explained in the write up: Carbon isotopes, you are what you eat.
Carbon isotopic records from carbonates are of interest to paleoceanographers because they help understand the functioning of the carbon cycle during earth history. In carbon isotopes in carbonates there is not much of a temperature effect, but there are there are complex local effects in addition to the global effect of different volumes of carbon being stored in an isotopically-light reservoir (biosphere).
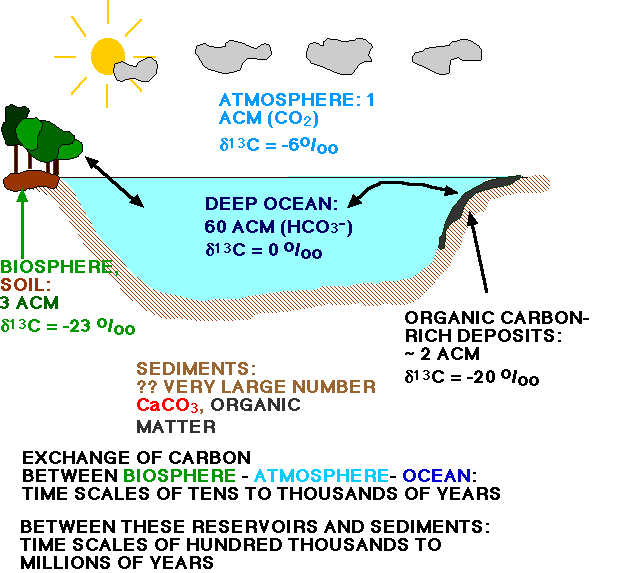
Figure: The various reservoirs of carbon on Earth (limestone, biota, CO2 in atmosphere) all have different carbon isotopic compositions. If more carbon is stored in one of these reservoirs, the isotopic composition of others reservoirs changes to reflect that storage. For instance: if there is more carbon stored in organic matter which is isotopically light, the average carbon composition of dissolved carbon in the ocean and carbon dioxide in the atmosphere will become heavier.

The two major sedimentary reservoirs of carbon are organic material and calcium carbonate. The precipitation of carbon in carbonate involves only very little isotopic fractionation relative to total dissolved inorganic carbon. The d13C of carbonate is relatively insensitive to changes in temperature (about 0.035‰ per oC). Therefore the d13C of inorganically precipitated carbonate in the oceans is very close to that of the total dissolved carbonate. The situation is more complex for organically precipitated CaCO3, because some organisms do not simply use dissolved bicarbonate, but their own metabolically influenced or produced CO2. We try to select organisms that do not show this behavior.
In contrast, there is very strong fractionation during the capture of carbon in organic matter by photosynthesis. The photo-synthetic fixation of carbon involves a very large fractionation. The simplified reaction for photosynthesis is:
6CO2 + 6H2O -> C6H12O6 + 6O2.
All carbon in organic compounds in the biosphere is ultimately derived from photosynthetically produced material, thus all organic carbon compounds in the biosphere are isotopically very light, largely as a result of kinetic effects during photosynthesis. Average d13C values of organic carbon in land plants vary according to the chemical pathway of photosynthesis: plants using the C3 pathway of photosynthesis (most higher plants) show maximum fractionation, and have isotopic ratios between -23 and -33‰, with an average of about -26‰, while the isotopic composition of CO2 in the atmosphere is about -6 to -7‰. Plants using the C4 pathway (most tropical and marsh grasses; possibly only since the Cenozoic) average about -13‰, ranging from -9 to -16‰. The photosynthetic reaction pathways of marine phytoplankton, which use dissolved CO2 (gas) are not well known, but the organic matter in marine phytoplankton shows a range of values between -10 and -31‰, with most values between -17 and -22‰.
There is a difference in fractionation during the formation of organic matter depending upon the temperature of the surface waters (in contrast to the lack of temperature effects during the formation of carbonate): at high temperatures the solubility of CO2 (gas) is low, thus there is only little available to the phytoplankton. Under these conditions the fractionation is less (the organisms are "less picky"), resulting in values of organic matter of as high as -13‰. At low temperatures, the solubility of CO2 (gas) increases, and thus its availability to the phytoplankton increases, resulting in much larger fractionation (values down to -32‰). The concentration of dissolved CO2 (gas) in surface sea water (and thus the amount of fractionation involved in the formation of organic material during photosynthesis) depends upon the concentration of CO2 in the overlying air as well as on the temperature. Thus, if we would know the carbon isotopic composition of organic matter we would know the fractionation, and if we would know the temperature, we could work out the CO2 concentration in the water, and thus in the overlying air. This method of using the carbon isotopic composition of organic material as a paleobarometer for pCO2 in the atmosphere is in the process of being worked out.
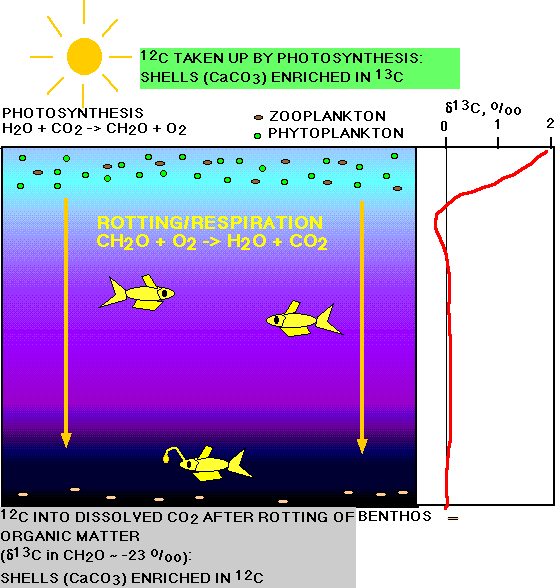
In general the photosynthetic activity in the oceans (limited to the
photic zone) results in a very strong depletion of the total
dissolved carbonate in the surface waters in
12C,
strong enrichment in 13C.
Planktonic organisms living in the photic zone, and forming
calcareous tests from dissolved inorganic carbon (largely
HCO3--
) in these surface layers, thus
use carbon that is enriched in 13C
(isotopically heavy) for the formation of their tests. Common values
recorded in the present oceans for planktonic foraminiferal tests are
about +2 to +3‰, while the average whole ocean
d13C
value of the total dissolved inorganic carbon is around 0.
FIGURE: Effects of the biological pump on carbon isotopic composition. Photosynthesis in surface water layers; respiration and rotting below where light can penetrate. The world-averaged d13C profile in the water column of the oceans is shown in red.
There are many problems in evaluating the values obtained from
planktonic
foraminifera. Many species of
planktonic foraminifera contain algal symbionts, and the dissolved
inorganic carbon used in test calcification may not representative of
ocean water, but of the metabolic processes within their cells. In
general, we see the largest deviation from equilibrium values in
young specimens, which grow fast: tests of young planktonic
foraminifera are usually lighter by several ‰ than the tests of
adults.
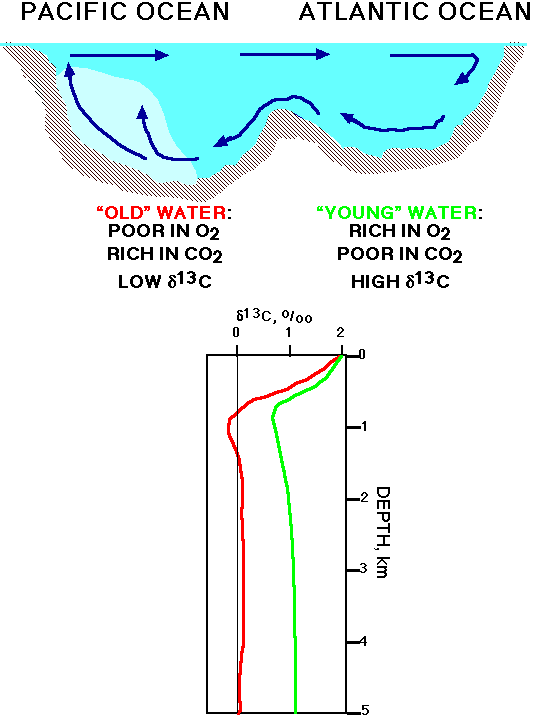
The oceanic record of d13C in planktonic and benthic foraminifera at any one site thus always reflects :
The d13C
value of whole-ocean TDIC has not been a constant over geologic time.
Presently over 90% of the carbon in the ocean-atmosphere system
resides in the deep ocean (the whole oceanic reservoir is about 60x
as large as the atmospheric reservoir in pre-industrial times), thus
we can use the d13C
signal from deep waters in the largest ocean, the Pacific, as a proxy
for the average ocean d13C
signature. The largest sedimentary reservoirs of carbon (output from
the ocean) are organic carbon and carbonate carbon. Presently the
outflux of carbon from the ocean in calcium carbonate is about 4 x as
large as the outflux in organic carbon. If there are changes in the
input or output ratio of organic matter versus carbonate carbon, than
the d13C
of TDIC in the whole ocean must change: if more carbon is removed
from the oceans in organic matter, then more
12C
is removed from the oceans, thus the d13C
value of TDIC in the whole ocean increases. Similarly, if there is a
period of strong erosion of organic material (e.g., coal) from land
and its influx in the oceans, then the average d13C
value of the TDIC in the oceans decreases.
The d13C values of all the dissolved carbon in the oceans have thus not been constant over geological time. The oceanic carbon reservoir (see carbon cycle model) is very much larger (about 60 times) than the atmospheric reservoir, and we can thus say that the "atmosphere is the slave of the ocean". Whatever happens to the isotopic composition of the oceans is transferred to the atmosphere. Changes in the sizes of these reservoirs occur slowly (on a geologic time-scale, 105 to 106 year), because oceanic deposition and erosion can not be re-organized quickly. For instance, a change in erosion rate may result from a change in continental elevation, i.e., mountain building processes (see figure below for a carbon isotopoc record for the Cenozoic). A reservoir that can react quickly (on the order of hundreds to thousands of years), however, is the biosphere. Presently, about 80% of all organic carbon is stored on land (and about 78% of all this material is present in soils), about 20% in the oceans (almost 90% of the organic carbon in the oceans is in the deep sea). We expect changes in the terrestrial biosphere mass to be reflected in the carbon isotopic composition of TDIC in the oceans via the d13C of CO2 in the atmosphere, because the "atmosphere is the slave of the ocean" as a result of its much smaller size.
In the d13C record of benthic and planktonic foraminifera we would expect a reservoir-change to be reflected in both planktonics and benthics, and thus no changes in the surface to deep gradient should occur IF there are no concurrent changes in productivity. Changes in oceanic productivity or deep-ocean circulation should result in a change in deep-to-surface gradient (i.e., the difference between the values for benthic and planktonic form).
The most severe case of a 'change in oceanic producitivity on short time scales' are the mass extinctions, such as the extinction at the end of the Cretaceous. During such extinctions the difference in carbon isotope values of bottom-dwelling organisms and floating organisms descrease or even disppears, because oceanic productivity collapses. In addition, the size of the total biosphere on Earth descreases, and the light carbon atoms bound in the biosphere are released by rotting and let loose in the oceans as dissolved carbon. This isotopically lighter carbon is then used by organisms building shells. We thus can use the carbon isotopic composition of carbonate to monitor the occurrence and severity of mass extinctions; after mass extinctions there is a small reservoir effects (lighter), and a collpase of differences between benthic and planktonic values.
This simple way of estimating the size of mass extinctions is made more complex, because large-scale erosion of organic material (e.g., erosion of coal deposits) can also add isotopically light carbon to the ocean-atmosphere system. We can distinguish between erosional events and mass extinction events in various ways. Mass extinctions happen rapidly, large changes in erosion take geologically long time (more than 100,000 years). In addition, we can see from the fossil record whether organisms went extinct globally.
See the write-up on Greenhouse World for more information on changes in the carbon isotope record and mass extinctions.
FIGURE: Record of oxygen isotopes and carbon isotopes over Cenozoic time, as measured in the shells of deep-sea bottom dwelling unicellular organisms (foraminifera). Oxygen isotopes show overall deep-sea temperature and global volumes of ice caps (see red scale below figure), carbon isotopes show overall storage of organic carbon in organic matter (if globally more carbon is stored in organic carbon, the record of carbonates moves to heavier values). Note the very short-term changes to extremely low values at about 55.5 million years ago (in the latest Paleocene), the time of the LPTM (Late Paleocene Thermal Maximum, recently re-named the Paleocene-Eocene Thermal maximum or PETM). Columns to the right show various climatic, tectonic and biotic events. Figure from Zachos, J. C., Pagani, M., Sloan, L., Thomas, E., and Billups, K., 2001. Trends, Rhythms and Aberrations in Global Climate 65 Ma to Present. Science, vol. 292, p. 686-693