What are clathrates?
Clathrates are also called gas hydrates. Hydrates were discovered in 1810 by Sir Humphrey Davy, and were considered to be a laboratory curiosity. In the 1930s clathrate formation turned out to be a major problem, clogging pipelines during transportation of gas under cold conditions. Gas hydrates, also called clathrates, are crystalline solids which look like ice, and which occur when water molecules form a cage-like structure around smaller 'guest molecules'. The most common guest molecules are methane, ethane, propane, isobutane, normal butane, nitrogen, carbon dioxide and hydrogen sulfide, of which methane occurs most abundantly in natural hydrates. Water crystallizes in the cubic system in clathrates, rather than in the hexagonal structure of normal ice. Several different hydrate structures are known, with one structure (known as Structure I) the most common. In this structure the cages are arranged in body-centered packing; the unit cell contains 46 molecules of water and up to eight molecules of methane [(CH4). 5.75(H2O)], but not all cages are occupied. If all cages would be occupied by methane, one cubic meter of solid hydrate could contain 170.7 m3 of methane gas at standard conditions of temperature and pressure. In nature, one cubic meter of hydrate turns out to contain up to 164 m3 of methane. Recently clathrates have received attention as a possible energy source, and as playing a role in large undersea slumps which could result in dangerous tsunamis, as well as in climate variability.
What is the origin of the methane in clathrates?
The methane in gas hydrates is dominantly generated by bacterial degradation of organic matter in low oxygen environments. Organic matter in the uppermost few cm of sediments is first attacked by aerobic bacteria, generating CO2, which escapes from the sediments into the water column. In this region of aerobic bacterial activity sulfates are reduced to sulfides. If the sedimentation rate is low (<1 cm/kyr), the organic carbon content is low (<1% ), and oxygen is abundant, aerobic bacteria use up all the organic matter in the sediments. But where sedimentation rates and the organic carbon content are high, the pore waters in the sediments are anoxic at depths of only a few cm, and methane is produced by anaerobic bacteria. This production of methane is a rather complicated process, requires the activity of several varieties of bacteria, a reducing environment (Eh < 400 mV), and a pH between 6 and 8. In some regions (e.g., Gulf of Mexico) methane in clathrates may be at least partially derived from thermal degradation of organic matter, dominantly in petroleum (e.g., Kvenvolden, 1998). The methane in clathrates typically has a bacterial isotopic signature and highly variable d13C (-40 to -100‰), with an approximate average of about -65 ‰ (Kvenvolden, 1993; Dickens et al., 1995; Matsumoto, 1995). Below the zone of solid clathrates, large volumes of methane may occur as bubbles of free gas in the sediments (Dickens et al., 1997; Matsumoto et al., 1996).
Where do clathrates occur naturally?
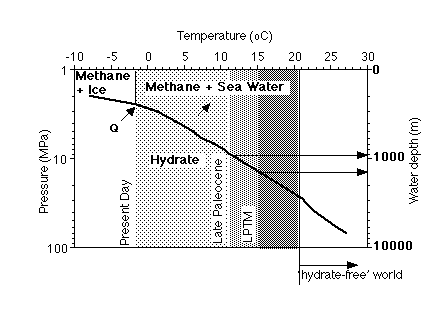
Clathrates occur wherever the conditions within the sediments are in the methane-clathrate stability field, and where methane and water are available (Fig. 1). This stability is limited by temperature and pressure: gas hydrates are stable at low temperatures and/or high pressures. Because of the requirements of pressure and temperature, and because of requirement of relatively large amounts of organic matter for bacterial methanogenesis, clathrates are mainly restricted to two regions: 1. high latitudes and 2. along the continental margins in the oceans (e.g., Kvenvolden, 1998). In polar regions the gas hydrates are commonly linked to permafrost occurrence onshore and on the continental shelves. In the oceans, gas hydrates are found on the outer continental margins, where the supply of organic material is high enough to generate enough methane, and water temperatures are close to freezing.
The average thickness of the clathrate stability zone along continental margins is about 500 m. The depth of its lower boundary is determined by the geothermal gradient: at greater depths in the sediment, the temperature becomes too high for gas hydrates. The thickest clathrate zones occur in regions of low geothermal gradients; most clathrates occur within 2000 m of the earth's surface.
Figure 1. Stability field (temperature and pressure) of methane hydrates (Dickens et al., 1995). Note the effect of warming by about 4oC from an initial deep water temperature around 11oC, leading to dissociation of hydrates over an ocean-wide zone of several hundreds of m thick.

How much clathrates are there?
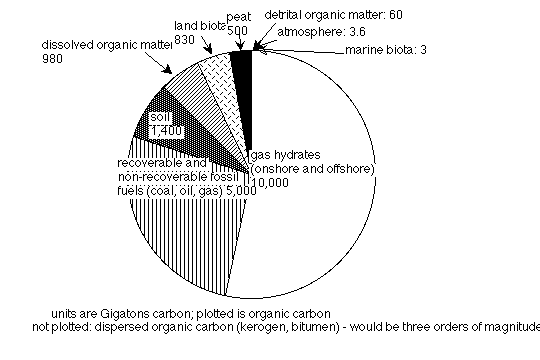
The extent of worldwide gas hydrate occurrences in the oceans has been evaluated using seismic exploration, because gas hydrates are characterized by the occurrence of an anomalous reflector parallel to the sea floor (Bottom Simulating Reflector or BSR), and cross-cutting ordinary sedimentary structures, because of the mismatch between the overlying high-velocity clathrates and underlying, low-velocity gas-bearing sediments (e.g., Kvenvolden 1988, MacDonald, 1990). The permafrost reservoir has been estimated at about 400 GtC in the Arctic (MacDonald, 1990), but no estimates have been made of possible Antarctic reservoirs. The oceanic reservoir has been estimated to be about 10,000 to 11,000 GtC (e.g., MacDonald, 1990; Kvenvolden, 1998). This oceanic clathrate reservoir is thus enormous (at almost a third of the size of the deep ocean reservoir; Fig. 2), and only small changes in its extent can have major effects on the atmospheric reservoir. Even the permafrost reservoir is on the order of hundreds of gigatons, not much smaller than the total amount of carbon in the terrestrial biosphere.
Figure 2. Sizes of organic carbon reservoirs (Kvenvolden, 1998).
Clathrates as possible fuel source
In the last few years, many governments (including those of the US, Canada, Russia, India and Japan) have become very interested in possibilities of methane hydrates as fossil fuels. On Thursday (13 April 2000) the U.S. Senate authorised $47.5 million over five years for the Department of Energy to study methane hydrates: ice-like crystals of methane said to have the energy potential equal to more than twice that of all fossil fuels combined. The House passed the bill earlier in the month, it now goes to the President, who is expected to sign it.
The realization that huge reservoirs of methane hydrates occur on the ocean floor and in permafrost regions (e.g., Kvenvolden 1988, 1993, 1998; Satoh, 1996) has led to exploration, mainly by oil-poor countries (e.g., Japan, India), and to recent efforts to try to find out how to use hydrates as energy source. These efforts are in their first stages: Japan just drilled a test hole in late 1999. The methane in gas hydrates might be recovered through injection of hot water or depressurization, but the process might turn out to be technically difficult and thus expensive. The USSR tried unsuccessfully to recover gas hydrates from permafrost reservoirs in the 1960s and 1970s. We do not know enough about the ways in which the gas hydrates occur in the sediments to be able to plan for their recovery, because hydrates have been drilled at very few places. If gas hydrates mainly occur thinly dispersed in the sediments, they will be difficult to exploit. Ocean Drilling Program drilling on Blake Ridge (offshore to the east of Charleston), however, suggested that our estimates of the total volume of gas hydrates might be too low rather than too high, and that large volumes of free methane gas might occur below the solid hydrates (Dickens et al., 1997). There are laso risks involved in trying to recover the methane (see below).
Clathrates as a cause of tsunamis
During the formation of gas hydrates, methane and water become immobilized within the sediment pore spaces. Because of the presence of these solids (instead of pore waters and gas), the sediment can not become consolidated because the water can not be expulsed with increasing overburden as more sedimentation occurs. Cementation of the sediments does not occur when pore spaces are filled with hydrates (solid ice) rather than with water, from which minerals such as calcite can be precipitated. Gas hydrate rich sediments are thus cemented by the hydrates, which may occupy much of the sedimentary section, but which are not stable when the temperature rises or the pressure falls.
This may lead to problems during continued sedimentation and further burial of the gas hydrates: the hydrates will become buried so deeply that the temperature will increase according to the regional geothermal gradient. The hydrates will then no longer be stable, and will disintegrate into a liquid water and gas mixture. The basal zone of the gas hydrate becomes under-consolidated, possibly over-pressured because of the release of the methane, leading to the development of a zone with low shear strength where failure could be triggered and massive landslides could occur. With the landslides, more gas could escape.
Several examples of possibly gas-hydrate linked extremely large slumps have been described, e.g., on the Norwegian continental margin (Bugge et al., 1987), where debris from the giant, three-part Storegga slide, over 450 m thick, is spread over a distance of 800 km. One of the Storegga slides caused a tsunami to deposit sediment up to 4 m above the high water line in Scotland (Nisbet and Piper, 1998). There are more of these mega slides in the same region (Laberg et al., 2000).
Clathrates of agents as climate change
Methane is greenhouse gas, and discharge of large amounts of methane into the atmosphere would cause global warming. It has been well documented that methane levels in the atmosphere were lower during glacials than during interglacial periods (e.g., Chapellaz et al., 1993; Blunier et al., 1995; Brook et al., 1996).
The dissociation of gas hydrates during deglaciation has therefore been linked to the ending of ice ages during the last the last few millions of years (MacDonald, 1990; Nisbet, 1990; Paull et al., 1991; Haq, 1998; Raynaud et al., 1998). Paull et al. (1991) suggested that the occurrence of large oceanic gas hydrate reservoirs are the factor limiting the severity of ice ages. During formation of large polar ice sheets sea level falls, reducing the pressure on the ocean margin gas hydrates. The shallower gas hydrate deposits become unstable, and release methane into the atmosphere, which causes warming and the ending of the ice age. This scenario remains questionable, however, because almost certainly the permafrost has hydrate reservoirs were much larger than now during glacial periods, when permafrost regions were much more widespread than they are now.
The scenario of large pulses of methane into the atmosphere might be checked by analyzing methane concentrations in polar ice cores. The methane peak concentrations in the atmosphere would not remain for long, because methane has an atmospheric residence time of only about 10 years, being oxidized to CO2. The time resolution of ice core sampling has until now not been enough to resolve such short-term peaks (Thorpe et al., 1996; Raynaud et al., 1998).
In addition, gas hydrate dissociation has been suggested as the cause for oceanic anoxia and massive extinctions of marine biota at the end of the Permian (Matsumato, 1995): oxidation of the methane within ocean waters could have used up a large part of the dissolved oxygen in the oceans. A much better documented possible episode of massive hydrate dissociation occurred during a short-term warming event occurred at the end of the Paleocene (Dickens et al., 1995, 1997; Matsumoto, 1995), when carbon isotopic values in the ocean-atmosphere system decreased by ~ -2.5 ‰ (see paper on reserved reading by Dickens et al., 1997).
Unique features of clathrates as part of the carbon cycle
Methane hydrates are part of the carbon cycle with unique features: they serve as a possible large source of isotopically extremely light carbon, but NOT as a sink. The carbon in methane hydrate was originally stored in the sediments (lithosphere, out of contact with the ocean and atmosphere) as organic carbon. Thus only carbon with the isotopic signature of organic carbon is taken out of the ocean-atmosphere reservoir - influencing the carbon isotopic value of the remainder in the well-known way (ratio of organic carbon to carbonate of sediments). Within the lithosphere, isotopically light carbon is strongly enriched in the methane in gas hydrates by bacterial action. The isotopically heavier remainder is isotopically not much heavier than 'normal' carbonate, and ends up in carbonate in the lithosphere. As long as both the methane hydrates and the remainder remain in the sediments there is no effect on the exospheric carbon reservoirs, but when the methane hydrates dissociate, material strongly depleted in the heavy isotopes escapes from the lithosphere into the ocean-atmospheric reservoir - making that reservoir lighter. The effect on the large sediment reservoir is not noticeable.
This means that the carbon that is stored in the methane hydrates was taken from the ocean-atmosphere system as carbon with an average isotopic composition of about -25‰, but the carbon returning to the ocean atmosphere system through the methane hydrate loop is isotopically ~ -60‰. Overall decreasing methane hydrate reservoirs (e.g., as a result of fluctuating sea levels during glacial -interglacial alternation) thus lead to a secular trend towards a lighter carbon isotopic signature of the ocean-atmosphere reservoir.
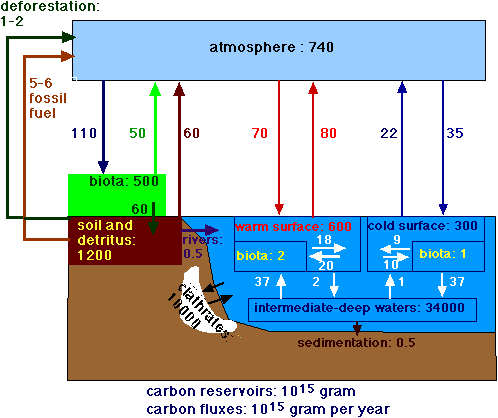
Compare Figure 3 A (Carbon cycle) with Figure 3B (Carbon cycle with clathrates).
Figure 3A.
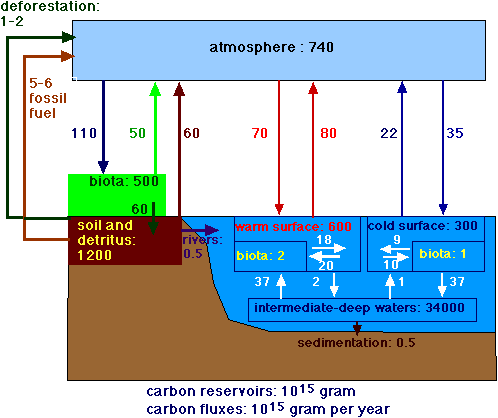
Figure 3B.
References