URBINO SUMMER SCHOOL IN
PALEOCLIMATOLOGY
2005
DEEP-SEA BENTHIC
FORAMINIFERA
Ellen
Thomas
Benthic foraminifera are an important component of the deep-sea
biomass in the present oceans, adapted to its cold, dark, and
extremely oligotrophic environments. Faunas are highly diverse, and
many species have a cosmopolitan distribution. In addition to their
interest as indicator species living in the largest habitat on earth,
their tests have been used extensively in isotope and trace element
analysis aimed at reconstruction of past environments. This section
is designed to introduce the basics of what we benthic foraminiferal
taxonomy, ecology and paleoecology and their use as a proxy for
interpreting the state of past oceans and climates, specifically
oceanic productivity and deep water oxygenation.
- Classification, ecology and biogeography
of modern forams
- What are foraminifera?
- Morphology/taxonomy
- Phylogeny
- Environmental indicators
- Limits of actualism?
- faunal turnovers, extinctions
- K/T boundary non event
- Palaeocene/Eocene Thermal Maximum
extinction
- Eocene-Oligocene and middle Miocene:
gradual turnovers
- Pleistocene
‘Stilostomellaextinction’
“The case of the three species of protozoan (I forget the
names) which apparently select differently sized grains of sand,
etc., is almost the most wonderful fact I ever heard of. One cannot
believe that they have mental power enough to do so, and how any
structure or kind of viscidity can lead to this result passes all
understanding.”
Charles Darwin,
letter to W.B. Carpenter, 1872
Why study deep-sea benthic foraminifera?
- Habitat covers a huge part of the world
(largest habitat on Earth)
- Habitat resistant to change -> IF
faunas reflect environmental changes (e.g., temperature) ->
global change
- Faunas highly diverse, ecological
theories may be tested (stability-diversity hypothesis,
species-energy hypothesis, patchiness hypothesis)
- Global extinctions in the deep sea: very
unusual events, during last 90 million years only one (55 Ma
ago)
- Need to understand how they make test if
you want to dissolved/analyze it
What is ‘deep-sea’? (van Morkhoven et al.,
1985)
- neritic = 0-200 m
- upper bathyal = 200-600 m
- middle bathyal = 600-1000
m
- lower bathyal = 1000-2000 m
- upper abyssal = 2000-3000
m
- lower abyssal > 3000 m
Note that
‘bathyal’ covers sites on continental margins as well as in
open ocean (sea mounts)
What is a ‘foraminifer’?
- Protist, network of
‘granuloreticulate pseudopodia’
- Complex life cycles (sexual and asexual
generations)
- Naked forms exist, commonly a
‘test’ (shell) is present
Kingdom Granuloreticulosa, Phylum Foraminiferida
Genetic information
indicates that unicellular eukaryotes, which were until recently
classified into the Kingdom Protista (Other Kingdoms: Kingdom
Prokaryotes, Kingdom Plantae, Kingom Animalia, Kingdom Fungi).
Nowadays we recognize the Superkingdoms Archaea and Eubacteria
(formerly Prokaryotes), and the Superkingdom
Eukaryotes (Protists, mushrooms,
plants, animals). The Superkingdom Eukarya is being reinterpreted,
with many group formerly placed together in “protists’ now
being elevated to the level of ‘Kingdom’. This means that
we now think that, for instance, foraminifera are as different from
other protists (e.g., dinoflagellates, radiolarians, diatoms) as cows
are from oak trees. Genetic information also strongly indicates that
Foraminifera together with test-less, freshwater forms form a
monophyletic group, which split off from other life forms early on,
maybe in the late Proterozoic.
Basic terminology for foram tests:
- Chamber: cavity containing
cytoplasm
- Chambers separated by septa; connected
by foramina (holes) in septa
- Foramen in last chamber is called
aperture
- External lines of junction of chamber
walls and septa: sutures
- Chambers enveloping earlier ones:
involute
- Chambers leaving earlier ones visible:
evolute
- Disk-shaped spiral where two sides look
the same: planispiral
- Disk-shaped spiral with one evolute, one
involute side: trochospiral
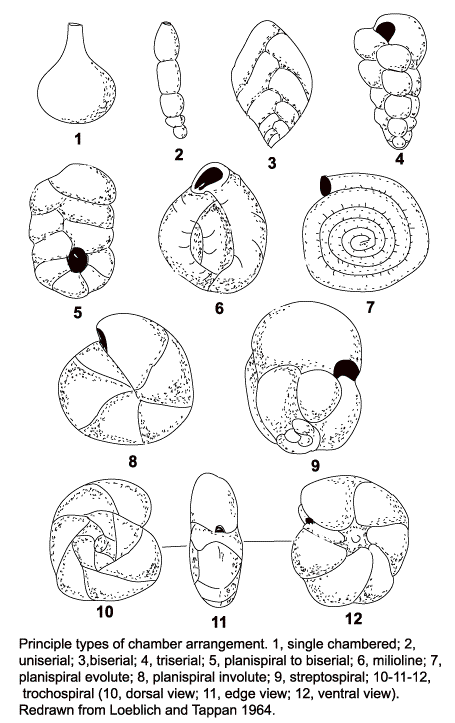
Chamber arrangements
- 1: single chamber (unilocular,
monothalamous)
- 2: uniserial
- 3: biserial
- 4: triserial
- 5: planispiral to biserial
- 6: milioline
- 7: planispiral evolute
- 8: planispiral involute
- 9: streptospiral
- 10-12: trochospiral
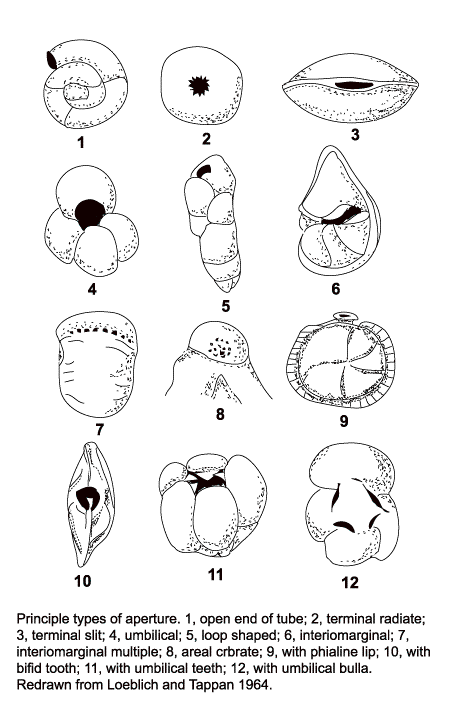
Apertures:
- 1. Open end of tube
- 2. Terminal radiate
- 3. Terminal slit
- 4. Umbilical
- 5. Loop shaped
- 6. Interiomarginal
- 7. Interiomarginal multiple
- 8. Areal cribrate
- 9. Phialine lip
- 10. Bifid tooth
- 11. Umbilical teeth
- 12. Umbilical bulla
Orders of Foraminifera (based on
wall structure and chemistry; B. K. Sen Gupta, ed., 1999, Modern
Foraminifera, Chapter 2 (p. 7-35), Kluwer Academic Publishers). (see
also practical)
- ALLOGROMIDA: organic wall, usually 1
chamber; Cambrian-Recent
- ASTRORHIZIDA:agglutinated, organic
cement, usually 1 chamber or branching tube;
Cambrian-Recent
- LITUOLIDA: agglutinated, organic cement,
many chambers, usually planispiral spiral;
Cambrian-Recent
- TROCHAMMINIDA: agglutinated;
organic cement, many chambers, usually trochospiral;
Cambrian-RecentTEXTULARIIDA: agglutinated, low Mg-calcite
cement; Cambrian-Recent
- FUSULINIDA: microgranular calcite; many
complex chambers; Silurian-Permian
- MILIOLIDA: high Mg calcite,
imperforate, many chambers (porcellaneous, no pores); miliolid
chamber arrangment; Carboniferous-Recent
- CARTERINIDA: low Mg calcite, hyaline,
pores or no pores; spicules, plani- or trochospiral;
Tertiary-Recent (?)
- SPIRILLINIDA: low Mg calcite; hyaline;
single crystal; spiral; Jurassic-Recent
- LAGENIDA: low Mg calcite,
hyaline; pores, 1 or many chambers, uniserial or planispiral;
monolamellar; Carboniferous-Recent
- BULIMINIDA: low Mg calcite;
hyaline; pores; many chambers; bilamellar; toothplate;
Triassic?-Recent
- ROTALIIDA: low Mg calcite;
hyaline; pores; many chambers; bilamellar; trocho- or
planispiral, annular, irregular; Triassic-Recent
- GLOBIGERINIDA: low Mg calcite
(aragonite in few extinct forms); pores; many chambers;
bilamellar; radial crystals (PLANKTON);
Jurassic-Recent
- INVOLUTINIDA: aragonite; 2 chambers -
2nd tube
- ROBERTINIDA: aragonite; pores; many
chambers; trochospiral; Triassic-Recent
- SILICOLOCULINIDA:opaline silica, no
pores; chamber arrangements as in miliolids;
Miocene-Recent
Genetic evidence suggests
strongly that Allogromida (‘naked’) and Astrorhizida
(agglutinated) are one order.
Phylogeny of deep-sea benthic foraminifera
- All common deep-sea groups today
(rotaliids, buliminids, lagenids, textulariids) and many of the
more common families and morphotypes within these groups have
existed in the deep sea (~>1000 m) since the Late Cretaceous (~
Campanian)
- Miliolids are dominantly warm, shallow
water forms, with few genera in the deep sea, since middle
Miocene
What is the function of the test of benthic
foramifera?
- Probably not support (small organisms in
water - no support needed)
- Probably not protection (many are
swallowed whole by predators, although some predators drill holes
in tests)
- Metabolism - get rid of salts? (but some
forms precipitate calcite from undersaturated water)
- Varying functions: keep nucleus/nuclei
protected, keep symbionts together, light for
symbionts
- Structures direct pseudopods - feeding
importance
Granuloreticulate pseudopods, the main distinguishing
character of foraminifera.
- Cytoplasm different from main mass
within test (endoplasm - ectoplasm)
- Granules are various organelles (e.g.,
mitochondria, microtubules, phagosomes)
- Main mass exits from aperture;
also protoplasm around test
- Anastomizing; bidirectional flow;
streaming process not understood
- Membrane, surrounding
microtubules
Pseudopodia: fundamental importance, mechanism through which
forams interact with environment
- Form complex ‘spiderweb’,
continually remodeling as while transporting material towards and
away from main body
- Motility, attachment, collecting
material, extruding material, feeding, exchange gases, chamber
formation, protection
- Digestion (partially)
What do forams eat? (almost everything)
- Herbivores: graze algae
- Passive suspension feeding (pseudopods),
e.g., C. wuellerstorfi
- Deposit feeding (very common in deep
sea)
- Ingest sediment, algal cells, bacteria,
organic detritus
- Carnivory (also multicellular
organisms); sticky pseudopods
- Parasitism (other forams,
molluscs)
- Uptake of dissolved organic
matter
- Endosymbiosis: algae, possibly bacteria;
kleptochloroplasts
- Many are selective feeders, e.g., fresh
phytoplankton, more degraded matter
Who eats foraminifera?
- In general: detritivores (non-selective:
take up mud with foraminifera and other food
particles)
- Mollusca, including various snails
(gastropods, e.g., Natica), juveniles of which drill holes
in tests
- Specialized foram eater: scaphopods
(Dentalium), elephant’s tooth shell
Reproduction: complex alternation of sexual-asexual
generations
- no males and females
differentiated
- variable how common sexual reproduction
is: many species have many asexual generations per sexual
generation
Note that the gametes may
exist in free form for at least several days, and function as
‘propagules’, i.e., help in spreading benthic forms
worldwide (hence many cosmopolitan taxa).
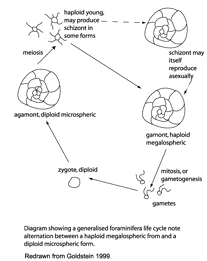
Test formation in forams:
(calcite,
hyaline)
- Endoplasm combines with pseudopods,
assumes shape of next chamber ‘anlage’
(logarithmic size increase), sometimes whole test surrounded by
cyst (collected grains, including sediment, algae,
etc.)
- Organic lining forms around
‘anlage’; pseudopods active
- Precipitation of calcite on one
(monolamellar) or both sides of lining (bilamellar) and over
earlier formed chambers
Foraminifera cover calcite
wall of earlier chambers, and walls between chambers are (in
bilamellar forms) existing of 4 layers (one from each adjoining
chamber), so that one should be careful in using spot-analysis
(laser-zapping) of foraminiferal subsequent chambers and septa in
foraminifera with the aim of using these analysis for very high
resolution records.
As an additional
complexity: some (porcellaneous) foraminifera have been shown to take
up a droplet of water within the cytoplasm, then use ions in the
‘internal pool’ to form thin craysallites within that
droplet, thus causing chemical/isotopic heterogeneity. Other use ions
from droplet, but appear to keep that droplet open to exchange with
sea water.
Some species (e.g., hyaline
Amphistegina) use ‘pooled ions’, others (e.g.,
porcellaneous Amphisorus) do not. We do not know whether these
are typical for the larger groups or not; at least another few small
hyaline species also used ‘pooled ions’.
What do we know about deep-sea forams?
Much early data on deep-sea
benthic foraminifera (and on other deep-sea groups) were collected on
the 1872-1876 Challenger
Expedition (benthic foraminifera
described by Brady, 1881, 1884). For updated taxonomy and
re-publication of plates see Jones, 1994.
Importance of deep-sea benthic foraminifera
- Widely varying estimates of total
oceanic foraminiferal biomass; up to 50% of eukaryotic biomass
(0.02 to 10 g/m2)
- More than
106/m2
- Opportunistic feeders: species are not
usually proxies for a simple environmental parameter (depth,
salinity, food supply, oxygen)
Fundamental niche:
species could theoretically exist under these condition
Realized niche:
species really exists under these conditions (smaller space than
fundamental niche)
Interpretation of deep-sea benthic
foraminiferal assemblages:
In the 1970s,
Lohmann first recognized that the water masses in the North Atlantic
(e.g., AntArctic Bottom Water, AABW, and North Atlantic Deep Water,
NADW) were characterized by typical foraminiferal assemblages
recognized in multivariate analysis (Lohmann, 1978). It turned out,
however, that it was not possible to typify global water masses by
faunal assemblages consistently, leading to disappointment in the
1980s. In the 1990s, however, many new studies of recent faunas were
directly or indirectly linked to the JGOFS (Joint Global Ocean Flux
Studies), and led to recognition of the importance of food in the
life of foraminifera: they depend upon food delivered from primary
productivity in the surface waters, 1000s of meters away.
Delivery of food to ocean floor:
Marine
snow: particles mm-cm sized,
consisting of dead and dying phytoplankton, zooplankton exoskeletons,
fecal matter). These fall at a speed of 102-103
m/day; a single unicellular alga would probably not even sink to the
sea floor, being re-suspended many times.
Seasonality of
productivity at pelagic mid latitudes: pulse of phytodetritus,
followed by rapid growth-reproduction of some benthic
foraminifera
Relatively
high, continuous supply along continental margins; there freshly
produced organic matter is augmented with more refractory organic
material derived from lateral transport
Food from surface to bottom:
Very little
(~1% or less) primary produced material reaches sea floor; follows
seasonal productivity (‘fresh phytodetritus)
Ballasted by
silica (diatoms), carbonate (foraminifera), dust; in fecal pellets;
in glutinous material (diatoms, cyanobacteria); in ‘giant balls
of mucus’, larvacean
(tunicate) houses; carrion falls (‘dead whales’); lateral
transport (refractory organic matter)
Discrepancy
between food requirements of faunas and supply in sediment traps:
faunas need more than what is delivered
In the present world
we thus see bentho-pelagic coupling, in which the benthic faunas
reflect what happens at the ocean surface where their food is
produced.
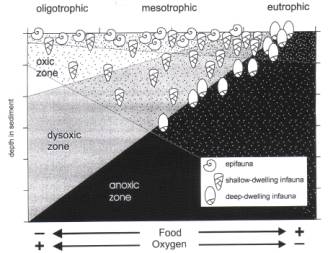
Jorissen et al., 1995: TROX
model (TR - trophic, food; OX - oxygen). Food is main
limiting/determining factor in low food regions, where all organic
matter is used up at the sediment/water interface. In such regions
there is no food for infaunal ) within sediment) species. In very
high food regions, the foraminifera and other organism do not eat
everything raining down, and the sediment pore waters become anoxic
(oxidation of some organic matter); here also foraminifera can not
live within the sediment. In mesotrophic regions foraminifera may
live down until 10-15 cm, with epifaunal, shallow infaunal, middle
infaunal and deep infaunal taxa.
Benthic Foraminifera in the deep oceans: is the present the
key to the past?
Can we use present benthic
foraminifera as source for proxy information on such environmental
parameters as temperature, salinity, depth, primary productivity,
oxygenation? If we can, is that information valid for
reconstruction of past environments?
Benthic Foraminifera in the DEEP oceans:
- Generally, not useful for age
determination (Late Cretaceous-Paleogene; early-middle Eocene;
Oligocene-early Miocene; middle Miocene-Recent
- Shelf - upper slope faunas are used in
biostratigraphy, as are larger benthic foraminifera (reefal
environments)
Benthic forams: what is proxy for what?
- Planktic/Benthic
ratio: paleodepth, dissolution,
surface productivity
- Benthic Foraminiferal
Accumulation Rate: surface
productivity
- Species % abundance,
Species
Diversity: paleodepth,
oxygenation of bottom waters, productivity, seasonality of
productivity, labile/refractory organic matter, water masses,
current activity, CaCO3 corrosivity
- ‘Morphotypes’:
microhabitats (infaunal/epifaunal) oxygenation,
productivity
1.
Planktic/Benthic
- Paleodepth: planktic forams not in
coastal zones (neritic), P/B >>100 in open ocean
- Dissolution: planktic forams fragment,
dissolve before benthics; deep-sea floor low P/B values indicate
depth below lysocline
- Surface productivity: more difficult,
but at higher food supply productivity (or: in shallower waters)
more benthic foraminifera
How to distinguish
planktic and benthic foraminifera:
- If there are zillions of them (in
absence of dissolution), they’re plankton
- There are MANY fewer planktic species,
so know your planktics (size fraction). In plankton, the chamber
form is inflated, the wall structure may be cancellate, aperture
is interiomarginal (but aperture may be covered, there may be
multiple apertures).
- Difficulties:
- Trochospiral forms : look at
aperture
- Biserial forms: look at aperture
(but there are forms, e.g., biserial genus
Streptochilu, looks very much like the benthic genus
Bolivina
2.
Benthic Foraminiferal Accumulation Rate
(BFAR): how does ‘food reaching bottom’ relate to
‘primary productivity’?
- Linkage between surface productivity and
quantity of bottom life (Herguera & Berger; number of
forams/m2/kyr; >150 mm)
- How much food reaches the sea floor: not
ONLY dependent upon productivity (water depth), not a linear
relation
- Lateral transport of organic matter
(focusing)
- Labile/refractory organic
matter
- Discrepancy between observations of
sediment community oxygen consumption and particulate organic
carbon (SCOC:POC)
3.a
Species % abundance
- Paleodepth: observation
(photosymbionts)
- Oxygenation of bottom waters,
productivity: very difficult to separate effects
- Seasonality of productivity:
‘phytodetritus species’, i.e., observation
- Labile/refractory organic matter:
observations, feeding experiments
- Water masses: observations
- Current activity: observations, shape of
foraminifera (‘tree-shaped’)
- CaCO3 corrosivity:
observations (Nuttallides umbonifera)
3b.
Species Richness, Diversity
- Deep-sea faunas: highly diverse, MANY
species rare, few species common (many benthic specimens needed
for analysis)
- Species richness: number of species
(number of specimens counted) - rarefaction techniques
- Various mathematical expressions of a
combination of number of species present and evenness of
distribution of specimens over species (e.g.,
Shannon-Weaver)
- Low diversity, high dominance (low
evenness): disturbed/not favorable environment
4.
‘Morphotypes’:
Infaunal/Epifaunal
- Can we determine mode of living from
shape of test ? E.g., infaunal epifaunal? Thus know the
‘microhabitat’ in which the foram lives? Example:
biserial = infaunal; trochospiral = epifaunal
- Partially, yes. Many exceptions, even
with present-day forams (Buzas et al., ‘93: assignments ~ 75%
correct)
- Foraminifera move through sediment
(follow food and/or oxygen gradients)
- Effects low oxygen (oxygen important
ONLY if <0.5 to 1.0 mg/L); Kaiho Benthic Foram Oxygenation
Index BFOI doubted (Kaiho, 1994, 1999)
How to define
infaunal and epifaunal:
Average Living Depth
(ALD10), Jorissen et al., 1995:
- Epifaunal/epiphytic: living above
sediment - water interface (rocks, plants)
- Epi/shallow infaunal: 0-1.5
cm
- Intermediate infaunal: 1.5-5.0
cm
- Deep infaunal: 5-10 cm
Example of difficulties: e.g., what is environmental
significance of faunas dominated by small, thin-walled
specimens?
- Low oxygen (difficult to separate high
food and low oxygen effects)
- Opportunistic growing (high food ->
early reproduction, rapidly varying circumstances)
- CaCO3 corrosive
Just on
faunas, not possible to decide which is most important factor in
specific case
Cenozoic benthic foraminiferal events:
- Non-event at K/T
boundary
- Extinction at P/E boundary
- Gradual turnover across oxygen isotopic
events Eocene-Oligocene and middle Miocene
- ‘Stilostomella’
extinction (1.2-0.6 Ma; Mid Pleistocene revolution)
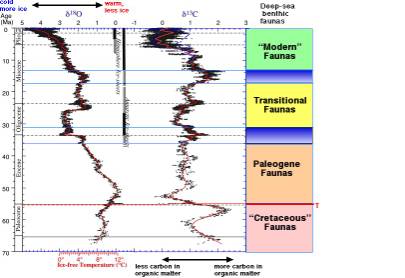
Note: diversity high
globally in greenhouse world, drops, and diversity gradient may have
been established at formation on Antarctic ice sheets (Thomas and
Gooday, 1996; Thomas et al., 2000). Various groups of common
deep-sea benthic foraminifera (Epistominella exigua, indicator
of fresh phytodetritus deposition; Nuttallides umbonifera,
indicator of AABW) only became common at Eocene-Oligocene transition
(establishment Antarctic ice cap.
What is typical in these non-analog ‘Greenhouse’
faunas?
- No ‘phytodetritus’ species
(opportunistic, seasonal growth blooms)
- Unserial lagenids, stilostomellids,
pleurostomellids (all long, then forms) buliminids-bolivinids
common in open-ocean settings; ‘high food’ taxa in
present oceans.
- Counterintuitive: at high temperatures,
metabolic rates faster, equal food supply would mean more
oligotrophic faunas
- Less common in open ocean species with
complex apertures (linked to pseudopod shape and streaming
behavior; feeding habits)
“Greenhouse Faunas Contradiction”:
- Benthic faunas suggest high food
supply
- Data from planktonic organisms suggest
lower productivity
- More efficient transfer of food to sea
floor? Different pattern of ocean circulation - Hay’s
eddies, rather than water masses?
- Different primary producers (e.g., more
diatoms? More sticky mucus, thus faster transport?
- Lower oxygen, thus less organic matter
degraded? (not very promising in few of data on present
Mediterranean, Red Sea)
- Primary productivity on sea
floor?
Higher primary productivity on sea floor
- Symbiontic chemosynthetic
bacteria
- Present day cold seeps: benthic
foraminiferal species that also occur elsewhere,
bolivinids/buliminids (high food)
- Forams living as cold-seep clams do; at
higher temperatures, bacteria higher metabolic rates thus higher
productivity
K/T boundary: no benthic foram extinction (Culver,
2003)
WHY
no serious consequences of collapse productivity’ on
food-starved deep-sea biota, in presence of bentho-pelagic
coupling?
- Less bentho-pelagic
coupling:
- Different ocean circulation, different
food transfer from surface to bottom
- More chemosynthetic productivity on sea
floorSurface productivity did NOT collapse: blooms of different
taxa
Paleocene/Eocene Benthic
Foraminiferal Extinction Event:
- ~ 30-50% species extinction; net
deep-sea extinction similar globally
- Drop in diversity (but in many places
affected by dissolution)
- Many cosmopolitan, large, heavily
calcified species extinct.
- Post extinction species dominance
patterns NOT the same globally: some places apparently more food,
some places apparently less food
- Post extinction faunas dominated by
small, thin-walled species
What caused the global benthic foraminiferal
extinction?
- Asphyxiation? (low oxygen). No
independent evidence for global anoxia-hypoxia (e.g., high organic
carbon, lamination), although in some places low oxygen conditions
did prevail (e.g., middle East).
- Starvation? Eutrophication? It seems to
be variable regionally; possibly more differences between highest
and lowest productivity values.
- Dissolution? Organic-cemented,
agglutinated foraminifera also show unusual faunal patterns
(Glomospira-peak), and extinction also occurs at the few
localities were dissolution is minor.
- Possibility: high global temperatures
caused metabolic problems &endash; productivity problems; needs
further investigation.
Cenozoic benthic foraminiferal faunas:
- Paleogene-Late Cretaceous community
structure of benthic forams may reflect different structure of
food supply to forams
- Possibility that transfer of food from
surface to bottom was different (ocean circulation?); or different
primary producers (diatoms?); or different seasonality
(less)?
- Possibility of greater importance of
chemosynthesis for food to forams
- Reorganization of faunas at cooling of
deep oceans reflects establishment of present-day food supply
structure -> more fresh phytodetritus to sea floor
- More reorganization in middle Miocene
(more cooling, expansion polar ice sheets)
- Last, by then rare, taxa typical for
earlier times extinct at Mid Pleistocene Revolution (Hayward,
2001)
My personal opinion on some points:
- Size fraction studied (prefer > 63
mm)
- Water masses in the present oceans
(temperature, salinity, oxygenation) are NOT determinants of
faunas (some properties of water masses, e.g., current speed, may
be)
- Foraminiferal paleobathymetry below
shelf depth and without information on specific region is NOT
accurate or precise
- Oxygen important ONLY if below values
~0.5-1.0 ml/L
- In most cases, interpretation of faunas
not easy (unless one feature of environment dominates)
- Present not necessarily key to
past
INTERACTION
BETWEEN MARINE BIOTA AND PHYSICO-CHEMICAL ENVIRONMENTS

