Overview lecture on the Earth's Climate.
What determines the Earth's Climate?
Long-term climate on Earth
Our planet has had an 'equable' climate over the last 4.5 billion years or so: we find no evidence for glaciation over the whole earth (oceans frozen solid, like on Mars), nor for great heat (oceans boiled and evaporated, like on Venus) even in very old rocks. We will discuss the nature of such evidence later in this class. Over all these billions of years the average global temperatures were probably not much different from those of today (within 10oC). This is really amazing: why was the Earth 'just right' for life as we know it? (Goldilocks problem). If we look at the amount of heat received from the Sun by Earth, Mars and Venus, we see that we can not solve the riddle by difference in distance to the Sun or size of the planet. It becomes even more amazing if we realize that many things were very different billions of years ago, when the Earth first formed from the solar nebula.
First, the Sun was not yet as hot as it is today. The amount of solar radiation reaching the Earth was only 70 to 80% of that of today, because nuclear reactions in a 'young' star give less energy; we know that from observations on stars of a similar size. This observation has been called the 'Faint Young Sun' problem: why did Earth not freeze over when the Sun gave so little warmth?
Second, the Earth's turned around much faster than today, because tidal friction resulting (largely) from the presence of the Moon had not yet slowed it down. Days may have been between 8 and 14 hours long. We know from growth rings in fossil corals that even as recently as 450 million years ago there were more than 400 days in a year. Such rapid rotation results in less transport of heat from the tropics to high latitudes: the winds (carrying warm air) are deflected to a larger degree from a North to South direction by the more rapid rotation of the Earth, and thus never reach high latitudes, as we will discuss later in this class. Therefore the poles may get so cold that ice caps form, which radiate heat back into outer space. Note that evidence on the chemical composition of Moon rocks, in combination with evidence from the way in which it moves together with the Earth around the Sun, indicates that the Moon was formed by the impact of a Mars-size asteroid on the early Earth, possibly at some time during the big meteor bombardment that ended about 4 billion years ago. The material from the core of the big asteroid was added to Earth, its mantle moved on and formed the Moon.
Third, more than about 3 billion years ago the continents were probably much smaller than today's, and there was thus more ocean surface. Oceans reflect back less heat, and more oceans would make Earth warmer. On Earth today about one third of the total heat received from the Sun is reflected back into space; changes in this amount can thus be of major influence on the Earth's climate.
But the fourth factor was the most important: the composition of the atmosphere. The atmosphere may have been made up dominantly of CO2 (compare: now there are only about 365 parts per million of the total atmosphere, before the industrial revolution there were about 280 ppm, then maybe more than 90%!). Even today the Earth would, on average, be as much as 35o C colder without the greenhouse effect of its atmosphere. We think that the early Earth's atmosphere had a composition dominated by CO2 because a planet the size of Earth would have lost its original atmosphere (primary atmosphere) which it took from the solar nebula (dominated by hydrogen gas, H2, with ammonia, NH3, and methane, CH4). These gases (especially hydrogen) are too light for a planet with the size of Earth to hold by its gravity. In addition, the primary atmosphere would have been blown away by the violent radiation of the Sun in its so-called T tauri stage. A secondary atmosphere would then be supplied by outgassing from the Earth's interior; either rapidly (big burp theory) or more gradual. If we look at what comes out of volcanoes now, we suppose that this secondary atmosphere consisted of dominantly CO2, with some dinitrogen gas N2, water vapor H2O, minor carbon monoxide CO, SO2, H2S. Of course, we need to look at the type of volcanoes which does not spew out lots of recycled crustal material: we look at volcanoes that appear to exhale material directly from the Earth's mantle and are located on oceanic crust on mid-oceanic ridges (e.g., Iceland, Hawaii). Note that the present atmosphere has about 80% N2, 20% O2; all that oxygen has been put into the atmosphere by organisms that photosynthesize (see below).
Where did all that CO2 go to? The outer parts of the Earth System (i.e., the exosphere) consist of the
Very little matter leaves the Earth: from the exosphere gases escape to outer space; matter moves from the exosphere into the endosphere (the interior of the earth) through subduction (plate tectonics). Little is added to the exosphere: cosmic dust and meteorites come in from outer space, while gases, volcanic ash, and liquid rocks (lava) come out of volcanoes into the exosphere from the endosphere. Within the exosphere matter moves continuously from one of the 4 'boxes' (atmosphere, hydrosphere, biosphere, lithosphere) into another. Motion goes rapidly within and into the atmosphere and biosphere (time scales of decades), slower into the hydrosphere (centennia in the upper ocean, millennia in the deep ocean), and very slow (on geological time scales, that is millions of years) into the lithosphere.
Over geologic time, life on Earth has succeeded in taking very large volumes of CO2 from the atmosphere (where it is a gas, with greenhouse properties and thus influences climate), and in depositing it into the lithosphere, where it is a solid and thus has no direct effects on climate. The carbon in the lithosphere is in the form of CaCO3 (limestone, thus skeletal material) and organic material (including soil humus, oil, coal).
Life takes CO2 from the atmosphere in the photosynthetic reaction used by all plants (including microscopic algae) to make organic matter from water and carbon dioxide:
H2O + CO2 <-> CH2O + O2 (Reaction 1)
CH2O is a short-hand way of saying 'organic matter. This reaction thus says: water and carbon dioxide are combined in photosynthesis to form oxygen and organic matter. The reaction moves from left to right during photosynthesis, from right to left during respiration and rotting, when organic matter decomposes back into its inorganic constituents. Another important reaction is the one according to which organisms secrete calcium carbonate (into the minerals calcite or aragonite, both with the formula CaCO3):
2 HCO3- + Ca2+ <-> CaCO3 + CO2 + H2O. (Reaction 2)
This reaction, the precipitation and dissolution reaction of carbonate, also can go both ways: from left to right during precipitation, from right to left during dissolution. Note that the notation of the units on the left hand side of the equation (with plus and minus signs) means that these are dissolved ions in water (the first ion is called bicarbonate, the second is calcium). These are the two most important reactions dealing with the interaction of the biosphere (life) and the lithosphere (rocks); note that the greenhouse gas carbon dioxide (CO2) participates in both reactions.
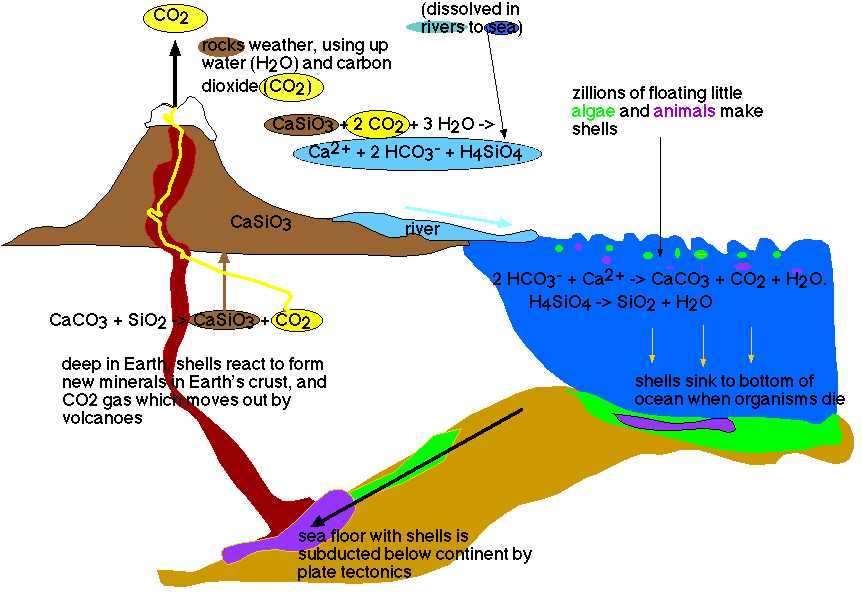
The Earth's thermostat (see figure)
The Earth is kept overall at a comfortable temperature by its build-in thermostat, which we can envisage as follows: CO2 gets into the atmosphere from volcanoes, and is used by chemical weathering reactions. If we take the average composition on Earth's crust as an average of limestone and siliceous rocks (CaSiO3), we can simplify this weathering reaction to:
CaSiO3 + 2 CO2 + 3 H2O -> Ca2+ + 2 HCO3- + H4SiO4. (Reaction 3),
or: rocks with water and carbon dioxide gives dissolved calcium, bicarbonate and dissolved silicic acid. These three dissolved components make it out to sea, eventually, in rivers. In the ocean there live numerous unicellular, photosynthesizing organisms, many of which make skeletons that consist either of calcite (CaCO3) or of opal (SiO2). The reactions are as follows:
2 HCO3- + Ca2+ -> CaCO3 + CO2 + H2O. (Remember ? Reaction 2)
H4SiO4 -> SiO2 + H2O (Reaction 4).
At least part of these skeletons ends up on the ocean floor, where they accumulate by the billions and form sediments (limestone with chert). At some time, maybe after millions and millions of years (the oldest ocean floor on Earth, in the South Pacific, is about 200 million years old), the sediments on their ocean floor are subducted under a continent, and reach depths to up about 600-700 kilometers below the Earth's surface. Then the whole mass is molten: sediments, igneous rocks, and some water that was trapped into them. The sediments are welded to the continent, while at least part of the denser oceanic crust descends into the mantle. Under the high pressures and at the high temperatures that prevail deep in the Earth's crust and mantle, the skeletons react together, according to the reaction:
CaCO3 + SiO2 -> CaSiO3 + CO2. (Reaction 5).
We are then back to the beginning of reaction 3: we have reconstituted 'average Earth's crust'. The CO2 is a gas, and will escape from deep in the earth's crust until it reaches the atmosphere again, and ready to participate in reaction 3.
Figure 1: The Earth's thermostat.

This cycle is indeed a thermostat, because it has several negative feedback loops (a process in which the results of an action counteract and thus stop the original action): if for one reason or other more CO2 reaches the atmosphere, the climate gets warmer, and then the weathering reaction runs faster (because there is more CO2 and because it runs faster at higher temperatures), but then the faster weathering takes the CO2 out of the atmosphere, so that the Earth cools. Positive feedback loops involve processes that by their results speed up the original action (and thus lead to disaster of some kind or another by a run away reaction): for instance, when the polar regions cool, more snow accumulates, which radiates more heat back into space, so that the polar regions cool even more, etcetera. Note that the thermostat depends upon the presence of abundant living organisms in the oceans: living organisms took the abundant CO2 from the atmosphere - where it exists as gas that influences the earth's climate, and stored it in the lithosphere. While over billions of years the Sun gave off more and more heat, the atmosphere lost more and more of its CO2, counteracting that effect.