|
|
|
|
Clays and asbestos: sheet silicates (phyllosilicates) and chain silicates (inosilicates)
Web sites with good models of silicate minerals:
Representations of silicate minerals:
We use three types of models to show mineral structure: polyhedral, stick-and-ball, space-fill. The stick and ball and space fill models show positions of atoms in the mineral. The polyhedral figures show the ribs connecting the centers of the atoms.
|
|
|
|
What are clays?
The word clay is used to indicate a rock or mineral type, but also a particle-size term, independent of composition.
Material in the clay size-fraction does not have to consist of clay minerals. For instance, quartz (SiO2) may occur in very small grains, in the clay size fraction. To prevent confusion between terms for composition and mineral structure (clay minerals) and size (clay-size) one should be explicit. In clastic sediment, usually most clay-size material does consist of clay minerals. The exception are clastic sediments of glacial origin: when glaciers physically grind down rocks, clay-size material may represent the mineral composition of the ground-down rock (rock flour). In biogenic pelagic sediments clay minerals commonly are rare in the clay size fraction, which consists mainly of fragments of diatoms (unicellular algae with e skeleton of non-crystalline SiO2) or platelets of calcareous nannoplankton ( unicellular algae with a skeleton of CaCO3).
Clay minerals are crystalline, i.e., the atoms in clay minerals are arranged in a regular order. Their crystalline nature was demonstrated in the 1920s, with the development of X-ray techniques. Before that time, some researchers postulated a crystalline nature (but could not prove it), others supposed that clay minerals were colloidal mixtures of fine-grained oxides.
Most of the common rock-forming minerals are silicates, and to understand their structure we have to understand what their building blocks are. Aluminosilicates usually consist of large and complex, chain-shaped or sheet-shaped crystals. The element Si thus behaves similarly to the element carbon in the large and complex organic molecules. In the latter, the carbon atoms share hydrogen atoms (basic unit: CH4); in the former, Si atoms share oxygen atoms (basic unit: SiO4).
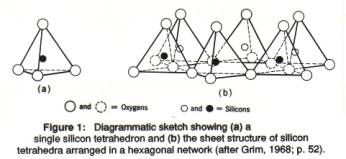
The basic building block of aluminosilicate minerals thus is the silicon-oxygen tetrahedron (figure 1), in which one silicon atom is surrounded by 4 oxygen atoms. A tetrahedron is a pyramid with a triangular base; the base triangle and the three side triangles are all congruent, equilateral triangles. In silicate minerals, a framework of silicon-oxygen tetrahedra forms the backbone, and other atoms or ions are fitted in according to their size and their electric charge, in the 'holes' in the framework. The whole mineral is kept together by chemical bonds between charged ions, but the resulting structure has a net neutral charge.
In different groups of silicate minerals the tetrahedra are linked together in different ways:
Clays are sheet silicates, asbestos minerals are either sheet silicates or chain silicates.
We will discuss the clay minerals as sheet silicates (phyllosilicates; after the Greek fullos = leaf), by first working out the 'ideal' basic framework of the minerals. Then we will see how the different parts of that framework are put together (stacking), and conclude by discussing different types of mineral construction (thus different minerals). Most naturally occurring minerals show complications: some atoms are replaced by others (substitution), and the framework may be distorted in different ways.
The structures of silicate minerals appear to be very complex, but becomes easier to understand if you keep track of two basic features while trying to build a mineral:
Substitution and distortion are the means to accomplish building a mineral while sticking to these rules.
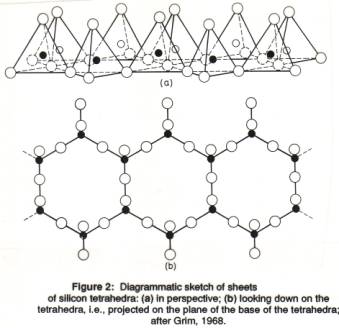
A single Si-O tetrahedron consists of 1 silicon atom and 4 oxygen atoms (Figure 1). The O-atoms are very much larger than the Si-atom; the O-atoms can be imagined as spheres stacked together, touching each other, with the Si-atom fitting within the hole left in the middle between the much larger O-atoms (as a marble between 4 tennis balls; see also space-fill figure above). Different types of silicates have different patterns in which the S-O tetrahedra are joined together. In sheet silicates the tetrahedra are organized in a two-dimensional, infinite layer in which the triangular bases of the tetrahedra are joined together. All the tops of the tetrahedra (the apical oxygen atoms) are at the same side of the sheet (sticking up; Figures 1b, 2). The morphology of the sheet silicates, usually thin platy crystals, reflects this underlying atomic arrangement.
A single Si-O tetrahedron consists of 4 O2- and one Si4+, which would give SiO44-, as for instance in silicic acid, H4SiO4. How to get charge balance? The Si atoms in a tetrahedra layer share the three corner oxygen atoms with another Si-atom, which results in (SiO2.5), usually written as (Si2O5)2-. Therefore, we see that the chemical formula of all sheet silicates has a section of (Si2O5)2-, with other elements added in.
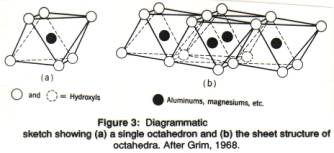
In sheet silicates, the tetrahedral layer is combined with another layer, in which the positive ions (cations) are surrounded by , and bonded to, 6 negative ions. If we draw an envelope around such a structure (one atom in the middle surrounded by 6 others) we obtain a shape called an octahedron (Figure 3). An octahedron looks like two pyramids with square bases joined together at the base. An octahedron has 6 corners: the four at the square pyramid bases, and the two at the top of each pyramid). A positive ion (e.g., Mg2+ or Al3+) is present in the center of the octahedron; at the 6 corner there are oxygen (O2-) of hydroxyl groups (OH-); rarely, OH- is replaced by a fluorine ion (F-).
The octahedra are combined in a layer, just as the tetrahedra were linked in layers, and the positive ions in their centers shares the negative ones on the corners (they share the edges of the octahedra). The corner of each octahedron, and thus each OH- group or O2- ion in an octahedral layer is shared by 3 octahedra (Figure 3 b). When the central ion is Mg2+, each possible central position is filled by that ion, so that the overall charge of the octahedral layer is zero. The central ion is bivalent (2+), and surrounded by 6 hydroxyls (OH-), total charge 6-. But each of the hydroxyls is shared by 3 octahedra containing an Mg2+, leading to a total charge per octahedron of -6/3=-2, just enough to neutralize Mg2+. When the central ion is Al3+, only two out of three possible central octahedral positions are filled, while the remaining 1 out of 3 are vacant. The mineral brucite, Mg(OH)2, consists of such linked octahedra with Mg2+ in the center and (OH)- at the corners. The mineral gibbsite, Al(OH)3, consists of layers of octahedra with Al3+ in the center (Figure 4) .
An octahedral layer in which all the centers of octahedra are filled with positive, bivalent ions (such as brucite) is called trioctahedral (because 3 out of 3 octahedral positions are filled); a mineral such as gibbsite in which only 2 out of 3 central octahedral positions are filled is called dioctahedral. The ways in which the vacancies are lined up vary from mineral to mineral.
Sheet silicates are formed by combining tetrahedral and octahedral layers. The octahedra are tied to the tetrahedra by sharing the oxygen atoms at the top of the tetrahedral layer; in these locations there thus will be an O2- instead of an OH- at the corner of the octahedron. In this way, we can combine one tetrahedron layer with one octahedron layer, and the resulting mineral is called a 1:1 or t-o mineral (the open sandwich type). But we can also put two tetrahedron layers on each side of one octahedron layer (hamburger type); such minerals are called1:2 or t-o-t minerals (see below). Thus we can subdivide sheet silicates into t-o and t-o-t minerals; each of these groups has then dioctahedral and trioctahedral members (see below). In nature, there are also mixed layer clays, in which sets of t-o and t-o-t layers occur, commonly alternating randomly.
Between the t- and o-layers is the interlayer space. In this space there may be additional positive ions (interlayer ions) to provide charge balance when the t- or o-layers are not in charge balance. Interlayer ions may be individual ions as in the micas, hydrated ions as in the clays, or even additional brucite-type layers.
One could build an 'ideal' t-o or t-o-t mineral if the tetrahedral and octahedral layers fitted together perfectly: they have to share the top-pyramid oxygen atoms in the tetrahedra. In reality, however, the two types of sheets to not fit perfectly, leading to many different types of distortions of the framework in order to adapt to the lack of fit. The positive ions present in the tetrahedral and octahedral layers determine how large the misfit is.
In order to make up a mineral, the layers must be stacked. We call the stacking direction the c-axis of the mineral. The b-axis is taken in a vertical plane, through the top and bottom points of the octahedra, parallel to the bases of the tetrahedra. The a-axis is (in an ideal, non-distorted structure), at right angles to the b-axis, and also parallel to the bases of the tetrahedra.
Ideal chemical composition and real minerals
Introduction
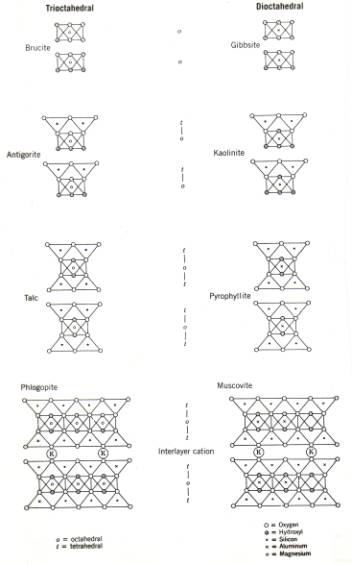
The schematics structure of several common minerals are shown in figure 4; their names, types and ideal formula are shown in Table 1.
Table 1: Formulas and names of minerals shown in figure 4.
Mg(OH)2 Al(OH)3 Mg3Si2O5(OH)4 Al2Si2O5(OH)4 Mg3Si4O10(OH)2 Al2Si4O10(OH)2 K(Mg,
Fe2+)3(Si3AlO10)(OH)2 K2(Al)2(Si3AlO10)(OH)2
Figure 4. Schematic development of some phyllosilicate structures; see also table 1.
Kaolinite
The common clay minerals ate hydrous alumino-silicates, and are located on the right hand side of Figure 4 and Table 1. The simplest clay mineral is kaolinite, a dioctahedral t-o mineral, with its name probably derived from the Chinese word kauling, meaning high ridge, the name of a hill near Jauchau Fu, China, where the material was mined centuries ago. The name kaolinite was used first in 1867 (S. W. Johnson and J. W. Blake, 1867, On Kaolinite and Pholerite. American Journal of Science, series 2, volume 43, p. 351-361.
The 'ideal' formula of kaolinite is Al2Si2O5(OH)4. Kaolinite analyses commonly give a composition close to that in that formula, i.e., about 46.54 weight % SiO2, 39.50 weight % Al2O3, and 13.96 weight % H2O. The structural units consist of one tetrahedral layer combined with one octahedral layer (Figure 5). Numerous of these t-o layers are stacked to make up the mineral grains.
In the stacking direction, the hexagonal rings of tetrahedra in two subsequent layers do NOT line up the center of one hexagon is shifted with respect of the center in the next layer. This results from the shape of the octahedral layer: the top and bottom triangle of the octahedron have their points in opposite directions, and the corners of the octahedra determine the position of the tetrahedral layer on top of the octahedral layer. The oxygen atoms in the base of the next tetrahedral layer are arranged in such a way that they can form (weak) hydrogen bonds with the OH-ions on the top surface of the octahedral layer.
Table 2: Ions in the kaolinite structure.
Layer Ion Charge of
layer Top octahedral
layer 3
(OH)- -3 Centers of
octahedra 2
Al3+ +6 Layer shared by
o-t 2O2-;
1(OH)- -5 Centers of
tetrahedra 2Si4+ +8 Base of
tetrahedra 3O2- -6 All
layers Al2Si2O5(OH)4 0
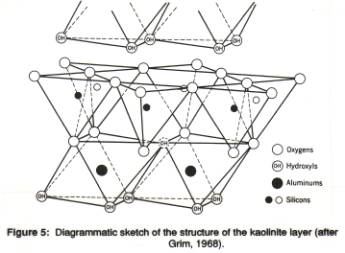
The structure of one kaolinite t-o layer is electrically neutral (uncharged). In many clays, however, ions (charged particles) attach themselves to clay surfaces in the so-called interlayer sites: the spaces between the t-o or t-o-t layer packages. The ions can become attached because of a net charge imbalance in the clay structure. In a neutral layer silicate such as kaolinite there is not much reason for either positive or negative ions to become attached to the mineral structure, and as a result there is very little substitution of other atoms for Si or Al. The t-o layers are bonded together only by weak intermolecular forces (van der Waals' bonds).
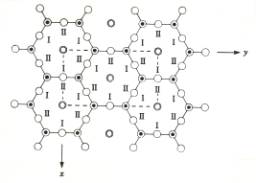
The vacancies in the octahedral layer in a dioctahedral mineral such as kaolinite (remember: only 2 out of each 3 centers of octahedra are occupied) are not randomly arranged. Usually these vacancies are arranged in such a way that two filled sites alternate with one vacant site (Figure 6). Note that there are 3 possible locations for the vacancy with regard to the tetrahedral layer. IF the octahedral layer did not contain vacancies, there would be only one way of stacking the t- and o-sheets, but with the regularly distributed vacancies present there are different possible mineral structures, differing only in the exact orientation of the 'lines of vacancies'. There are three different ways in which the t-o layers can be stacked, each resulting in a different mineral. These three different minerals have the same chemical formula, are all included in the kaolinite group, and differ only in the vertical pattern of arrangement of the location of the vacancies. Their names are kaolinite s.s., dickite and nacrite.
Figure 6. Top: organization of vacancies in the octahedral layer as seen from above. Each c and n indicates an empty or filled center of an octahedron, with n indicating a center with an Al3+ ion, c indicating a vacancy. Bottom: possible positions of octahedral cations above a tetrahedral sheet.
nncnncnncnncnncnncnncnncnncnncnncnnc
ncnncnncnncnncnncnncnncnncnncnncnncn
cnncnncnncnncnncnncnncnncnncnncnncnn
nncnncnncnncnncnncnncnncnncnncnncnnc
ncnncnncnncnncnncnncnncnncnncnncnncn
cnncnncnncnncnncnncnncnncnncnncnncnn
nncnncnncnncnncnncnncnncnncnncnncnnc
ncnncnncnncnncnncnncnncnncnncnncnncn
cnncnncnncnncnncnncnncnncnncnncnncnn
nncnncnncnncnncnncnncnncnncnncnncnnc
ncnncnncnncnncnncnncnncnncnncnncnncn
cnncnncnncnncnncnncnncnncnncnncnncnn
nncnncnncnncnncnncnncnncnncnncnncnnc
ncnncnncnncnncnncnncnncnncnncnncnncn
cnncnncnncnncnncnncnncnncnncnncnncnn
nncnncnncnncnncnncnncnncnncnncnncnnc
The minerals of the kaolinite group do not swell up in water: the polar water molecules find no reason to attach themselves to the neutral kaolinite structural unit. Not many ions are present in the interlayer sites, and therefore kaolinite is not a good cation exchange medium (see below). Kaolinites make poor soils, because there are no loosely bound ions present that plants need (e.g., K+). Kaolinite usually forms under low pH conditions, in acidic soils and in hydrothermal spring regions.
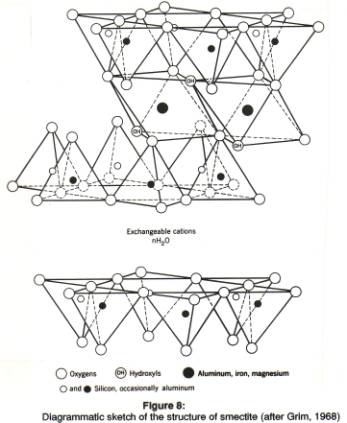
The ideal structure of the minerals in the smectite group is shown in figure 4 and table 1, under the name of the mineral pyrophyllite. Pyrophyllite is not usually classified as a clay mineral: it is a t-o-t dioctahedral mineral, with the 'ideal' formula of Al2Si4O10(OH)2. Note the difference in formula as compared to the t-o mineral kaolinite with the formula Al2Si2O5(OH)4. Pyrophyllite contains relatively more Si4+ and more O2- atoms than kaolinite, and fewer OH- ions, because of the added t-layer. The clays of the smectite group have the same ideal formula as pyrophyllite, but they always differ from the 'ideal' structure. These differences are caused by substitution of ions for the Si and Al atoms in the centers of the tetrahedra and octahedra, and by compensation of the resulting charge imbalance by addition of interlayer cations.
The Si4+ in the center of the tetrahedra may be replaced by Al3+, so that Al is present in the t- as well as in the o-layer. This means a charge imbalance, because Al is 3+, Si 4+. Up to about 15% of all Si ions may be replaced by Al ions. Quite a few different ions may substitute for Al3+ in its octahedral site, for instance Mg2+, Zn2+, Fe2+, Fe3+, Ni2+, Li+. When a bivalent (2+) ion substitutes for trivalent Al the charge can not be completely balanced by filling the vacant octahedral positions: as far as we know, a few positions may be filled, but the mineral remains dioctahedral, i.e., almost 1 out of each 3 octahedral centers is vacant.
In smectites the overall mineral structure is thus always imbalanced: there is no possible canceling of substitutions in the o-layer by substitutions elsewhere in the structure. Usually, the imbalance is from -0.2 to -0.6 per unit cell. To make up for this net imbalance, additional positive ions must be fitted somewhere in the structure. These cations are thus NOT present within the tetrahedra or octahedra, and are much more loosely bound in the crystal than the ions in the centers of these tetrahedra or octahedra. The additional positive ions are usually located between the t-o sheets. Some fit in the central hexagonal opening the rings of tetrahedra. The most common interlayer atoms are K+, Na+ and Ca2+.
The interlayer atoms are fairly loosely attached to the mineral structure, and therefore can be exchanged for other cations; they are therefore called exchange cations. For instance, if a smectite clay with mainly Ca2+ ions comes in contact with sea water, they clay minerals loose Ca2+ and take up Na+.
Cation exchange is a very important process in nature, and it partly regulates the composition of natural waters by absorbing and desorbing ions. The cation exchange capacity (CEC) is a measure of the charge imbalance of a clay, and a measure of the number of ions that can occupy the interlayer sites. The CEC varies, dependent upon the nature of the absorbed ions present in the interlayer sites. Kaolinite has a very low value of CEC, whereas smectites may have large values (70-130 meq/100g). The polar water molecules can also absorb onto the clay surfaces, because their positive sides will be attracted by the part of the clays with negative charge imbalance. Water molecules may also enter the interlayer spaces, leading to swelling of clays. The amount of water that can be absorbed depends upon the nature of the adsorbed ions in the interlayer spaces, especially their charge. When Na+ ions are abundant (as in sea water), large amounts of water can be taken up, leading to very strong swelling of the clays. Swelling of clays may destroy the loose structure of a good soil: invasion of salt water into agricultural regions thus leads to destruction of the physical properties of the soils as the result of Na+ adsorption, followed by abundant water absorption. In engineering geology, the nature of clays (t-o or t-o-t mineral structures) is thus crucial for the evaluation of such factors as slope stability during the construction of roads and bridges.
Smectite clays are formed under more alkaline soils conditions than kaolinite. Smectites well in the presence of water, and also in the presence of many organic liquids. There are very many different names for minerals in this group, and usage varies. The group is also called the montmorillonite group, especially in the older literature. Presently, the name montmorillonite is used for one mineral ion the group, with the overall formula Na0.66Si8(Al3.34.Mg0.66)O20(OH)4. Such a formula should be read as follows: in the octahedral position 0.66 out of every 4 Al3+ are substituted by Mg2+; this is balanced by the presence of Na+ in the interlayer position. The Si4+ in the tetrahedral positions has not been substituted. If there is substitution of Al3+ in the t-layer, the mineral is called beidellite. In the mineral nontronite there is substitution of Al3+ in the t-layer, and substitution of Fe3+ for Al3+ in the o-layer, with charge balancing by Na+.
The micas are a group of minerals with close family ties to the clays (Figure 4, table 1). They are t-o-t sheet silicates, but the separate triple sheets are tightly bound together by fixed interlayer cations that fit snugly in into the hexagonal holes in the bottom of the t-layer. The bonding by these interlayer cations (commonly K+), is made possible through the regular substitution of Al3+ for Si4+ ions in the tetrahedral sites: for each 4 Si ions, one is replaced by Al. To make up for the charge deficiency, the minerals builds K+ ions into the interlayer sites, and the t-o-t sheets are bonded together by these interlayer ions. The interlayer sites in micas are part of the structural framework of the micas, and the K+ ions at these sites are tightly bound and not as easily interchanged as those in the smectite clays. Micas are therefore much stronger than clays, where the t-o sheets and t-o-t sheets are bound together by weak van der Waals' forces. Micas thus can form large, extended sheets. Illite is a fine-grained variety of muscovite (white) mica group, the dioctahedral mica group, and is usually classified as a clay mineral. The trioctahedral member of the family is called phlogopite, more commonly biotite, in which some Mg2+ has been exchanged for Fe2+ (Table 1).
The trioctahedral counterpart of kaolinite has the formula Mg3Si3O5(OH)4; minerals with this chemical composition belong to the serpentine group. The two most common minerals in this group are called antigorite and lizardite. Both are fine-grained, fairly soft, and 'soapy' feeling minerals (soapstone). The third mineral in this group, however, with the same chemical composition and the same t-o trioctahedral structure is called chrysotile, and looks very different from the other two minerals. Chrysotile is asbestiform, i.e., it consists of very thin fibers. More than 90% of commercially used asbestos is chrysotile. The largest producer was Canada, with very large mines in Quebec (Thetford region). Chrysotile asbestos is very strong, a good heat isolator, resistant to corrosion by alkalis, but not very much to acids. It is usually not a good electrical isolator, because it contains Fe-rich impurities.
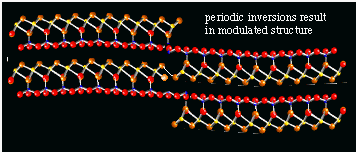
It is very unusual for a sheet silicate to form fibers. The other types of asbestos (see below) are all chain-silicates, in which the long chains of tetrahedra from the thin fibers. The markedly different properties of chrysotile as compared to the other serpentine-group minerals is caused by its remarkable mineral structure. The tetrahedral and octahedral layers do not really fit exactly on top of each other: the repeatable unit in the octahedral layer is just slightly larger than the repeatable unit in the tetrahedral layer. In most sheet silicates this discrepancy in size results in the formation of very small rather than large crystals. The crystals can not grow to large proportions because the size differences in the sheets add up, and the crystal just can not grow any further when the t- and o-layers are too much mis-aligned. In chrysotile, however, the sheets do grow large, but they are curved, and chrysotile asbestos fibers thus consist of small rolls of t-o sheets. A mass of chrysotile thus can be split into smaller and smaller fibers. Until the thinnest possible fiber (called fibril) is obtained. These fibrils are the rolls, with a fairly uniform diameter of about 2000 nanometer (10-9 m, figure 7).
Figure 7: transmission electron micrograph of a chrysotile fibril

In antigorite (see below), in contrast, the crystals grow into a sort of corrugated structure, in which about 17 tetrahedra are linked to about 16 octahedra, and the top-bottom direction of the sheets alternates every 17 tetrahedra.
What is asbestos? What other mibnerals than chrysotile can be
asbestiform?
Which are the 6 asbestos minerals?
Almost all industrially used asbestos consists of the first 3 minerals (bold). In the US, >95% is chrysotile, mainly mined in Quebec (Canada)
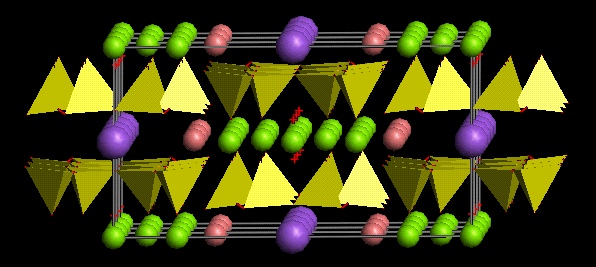
Structure of amphiboles
Amphiboles are double chains of silicon tetrahedra (in which Al may substitute for Si in the tetraedra), in which two tetrahedra-chains are linked together by an octahedral chain. In the octahedral chain there are many different possible positive ions present. There may be additonal positive ions outside the octahedral layer, in the large hexogonal space within the tetrahedral layer. These ions are similar to interlayer cations in clays.
Basic formula of an amphibole (see figure below):
A0-1M42M133M22T8O22(OH)2
The letters in this generalized formula show positions in the structure which then can be filled by different ions:
What are the fibers in amphibole asbestos?
NOT the double chains; they are much smaller than the fibers
Maybe fibrils are delimited by 'mistakes' in the way on which the chains are stacked; maybe fibrils are micro-crystals, slightly rotated with respect to each other
ALL asbestos minerals have non-fibrous counterparts (same composition)
What diseases are associated with asbestos?
In addition, asbestos exposure is associated with excess mortality due to cancer of the larynx and cancer of the gastrointestinal tract. The malignant diseases (the cancers including mesothelioma)are often fatal within a year or a few years of initial diagnosis, which usually 10-45 years after the exposure to asbestos. In contrast, asbestosis deaths typically occur only after many years of suffering from impaired breathing. Impaired breathing may also lead to heart disease.
WHY do these diseases develop?
We do not know exactly how asbestos fibers cause cancers. We do know that microscopic fibers can become airborne during various industrial processes or from handling of asbestos-containing materials and can then be inhaled and/or swallowed. As much as 50 percent or more of inhaled asbestos fibers can remain lodged in the lungs, where it is almost impossible for the body to eliminate them. Asbestos fibers are extremely resistant to destruction in body fluids, and many of these fibers are too long to be engulfed and removed by the cells that normally scavenge and remove particles that happen to deposit in the lungs.
Fibers, diameter several hundreds of nanometers (1 nm = 10-9 m). Penetrate lungs, active part (alvoles). Particles with a diameter of less than 2.5 mm (10-6 meter) can penetrate the alveoles efficiently. Asbestos fibrils: a few thousands nanometer in diameter, although length is much greater. Penetration depends upon diameter of fiber, not length.
Once in alveoles: fibrils can not leave. The white blood cells (roughly spherical) can not move the long fiber. The asbestos does not dissolve. Fibers thus accumulate in the lungs. In asbestosis, the lungs are so full of fibers that the latter loose their elasticity.
Generally, as the burden of
retained fibers increases in the body, so does the likelihood of
disease. Most asbestos-related diseases, particularly the malignant
ones, have long latency periods often extending 10-40 years from
initial exposure to onset of illness. While asbestos-related lung
cancer and mesothelioma are frequently not curable, they and other
asbestos-related diseases are clearly preventable by eliminating or
limiting exposures to asbestos. The amount and duration of exposure
are factors that can determine the risk of adverse health effects.
Asbestos: since when know to
humans?
Since when has it been known to cause disease ?
Very difficult to estbalish whether something causes cancer (is a
carcinogen)
Asbestos: one disease is related to exposure of asbestos (or similar minerals) and not to smoking: Mesothelioma. A cancer of the lining of lung or abdomen.
Different types of asbestos have different risk of developing mesothelioma, maybe because the risk depends on fiber diameter. :
>90% asbestos used chrsyotile. It is difficult to replace chrysotile in many applications (e.g., brake lining). There thus is an ongoing discussion on whether one should stop using chrysotile or not. Defective brakes are also killers. Maybe a replacement material is just as dangerous to health (if also fibrous), and it would take decades to establish that (click here for discussion of dangers of use of chrysotile).
Still a point of discussion: in how far is asbestos a public
health problem, rather than a professional health
problem?
Direct and indirect professional exposure well proven (clothing of workers washed at home). People who sells asbstos productes; kids, wives of asbestos workers; farmers on soils with asbestos. Exposure limites: 2 fibers/cc, > 5 mm length.
But air in cities contains common asbestos fibers, >30 times at country levels. Found in lungs of city dwellers everywhere. Do these low levels pose a health risk (above backgroud levels)? This is difficult to say, because dose-response relation ship are hard to establish: different people are sensitive at different levels : at what levels do people die? At what level do 50% of the rats exposed die?
Sampling: how to sample for asbestos? One has to use filters, but the
filters must have very small holes or the fibrils will go through.
Filters with very small pores let very little air through, so that
sampling goes very slowly. One needs to collect enough air to
establish low levels of asbestos fivers present.
How to identify asbestos?Large chunks are easy to identify, but it is the thinnest fibers which pose the health risk. One thus needs a procudre to recognze veru small amounts of fibrils. X-ray diffraction does not work for these small amounts. Phase contrast microscopy (using light microscope): difficult to see/recognize/count small fibers. Transmittent Electron Microscopy: very expensive, takes very long to study even one small sample.